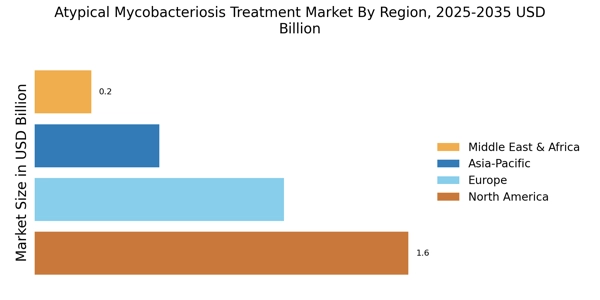Growing Investment in Research and Development
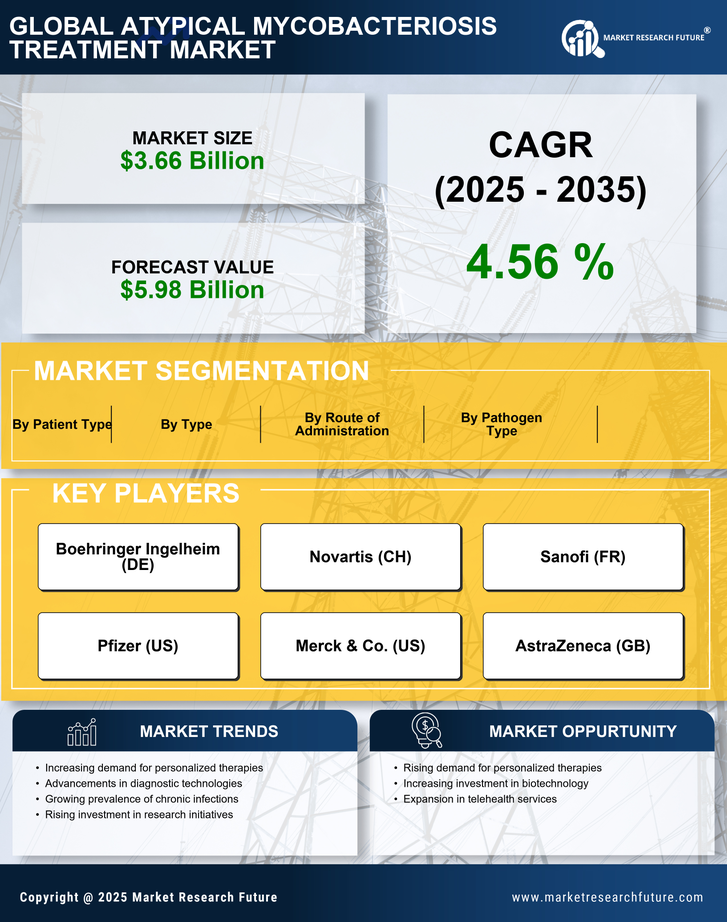
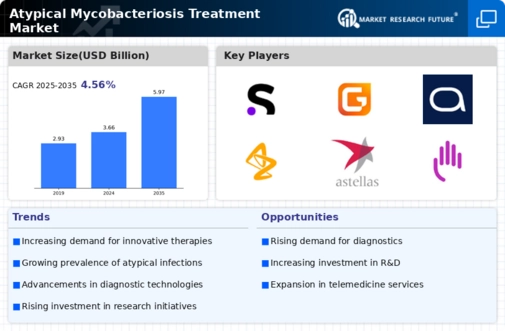
Growing investment in research and development is a crucial factor propelling the Atypical Mycobacteriosis Treatment Market. Pharmaceutical companies and research institutions are increasingly allocating resources to explore novel therapeutic approaches for atypical mycobacterial infections. This trend is evidenced by the rising number of clinical trials focused on new drug formulations and treatment regimens. According to recent data, the investment in R&D for infectious diseases, including atypical mycobacteriosis, has seen a marked increase, reflecting a commitment to addressing unmet medical needs. As these investments yield promising results, the market for atypical mycobacteriosis treatments is poised for significant growth, potentially leading to the introduction of innovative therapies.
Technological Advancements in Diagnostic Tools
Technological advancements in diagnostic tools are significantly influencing the Atypical Mycobacteriosis Treatment Market. Enhanced diagnostic capabilities allow for earlier and more accurate detection of atypical mycobacterial infections. Innovations such as molecular diagnostics and next-generation sequencing are becoming more prevalent, enabling healthcare professionals to identify infections more efficiently. This increased diagnostic accuracy not only facilitates timely treatment but also contributes to better patient outcomes. As a result, the demand for effective treatment options is likely to rise in tandem with improved diagnostic technologies. The integration of these advanced tools into clinical practice may also lead to a more comprehensive understanding of the disease, further driving the market.
Rising Awareness Among Healthcare Professionals
Rising awareness among healthcare professionals regarding atypical mycobacterial infections is a vital driver for the Atypical Mycobacteriosis Treatment Market. Educational initiatives and training programs are being implemented to enhance the understanding of these infections, particularly in regions where they are less recognized. As healthcare providers become more knowledgeable about the symptoms and treatment options, the likelihood of early diagnosis and appropriate management increases. This heightened awareness is expected to lead to a greater demand for effective treatment solutions. Furthermore, as more healthcare professionals engage in discussions and research about atypical mycobacteriosis, the market is likely to benefit from a more informed approach to treatment.
Increasing Incidence of Atypical Mycobacteriosis
The rising incidence of atypical mycobacterial infections is a primary driver for the Atypical Mycobacteriosis Treatment Market. Reports indicate that the prevalence of these infections has been steadily increasing, particularly among immunocompromised individuals. This trend necessitates the development and availability of effective treatment options. As healthcare providers become more aware of atypical mycobacteriosis, the demand for targeted therapies is likely to grow. Furthermore, the World Health Organization has noted a concerning rise in cases, which may lead to increased funding and research initiatives aimed at combating these infections. Consequently, the market for atypical mycobacteriosis treatments is expected to expand as healthcare systems adapt to this growing challenge.
Emerging Markets and Increased Access to Healthcare
Emerging markets are playing an increasingly important role in the Atypical Mycobacteriosis Treatment Market. As healthcare infrastructure improves in these regions, access to diagnostic and treatment options for atypical mycobacterial infections is expanding. This trend is particularly evident in countries with rising healthcare expenditures and a growing middle class. Increased access to healthcare services is likely to result in higher rates of diagnosis and treatment of atypical mycobacteriosis. Additionally, as pharmaceutical companies seek to penetrate these emerging markets, they may introduce more affordable treatment options, further driving market growth. The combination of improved healthcare access and targeted therapies could significantly impact the management of atypical mycobacterial infections.