Advancements in RNA Technology
Technological advancements in RNA-based therapies are transforming the Antisense Therapy Market. Innovations in RNA chemistry and delivery mechanisms have enhanced the efficacy and safety profiles of antisense oligonucleotides. For example, the development of modified nucleotides has improved the stability and binding affinity of these therapeutic agents. As a result, the market is witnessing a surge in the number of clinical trials and approvals for antisense therapies. The increasing investment in RNA technology is expected to drive the market, with projections indicating a potential market size of USD 4 billion by 2027. This trend highlights the critical role of technological progress in shaping the future of the Antisense Therapy Market.
Growing Focus on Rare Diseases
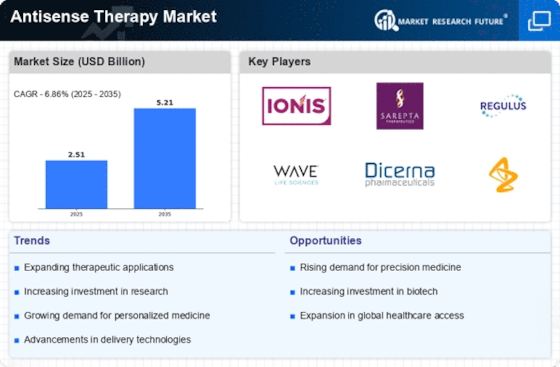
The increasing focus on rare diseases is significantly influencing the Antisense Therapy Market. With a growing number of therapies being developed for conditions that affect small patient populations, antisense therapies are emerging as viable treatment options. Regulatory agencies are providing incentives for the development of orphan drugs, which has led to a surge in research initiatives targeting rare genetic disorders. The market for antisense therapies aimed at rare diseases is expected to expand, with estimates suggesting a market value of USD 2.5 billion by 2025. This emphasis on rare diseases not only fosters innovation but also enhances the visibility and acceptance of the Antisense Therapy Market.
Regulatory Support and Incentives
Regulatory support and incentives play a crucial role in the growth of the Antisense Therapy Market. Governments and regulatory bodies are increasingly recognizing the potential of antisense therapies, leading to streamlined approval processes and financial incentives for developers. Initiatives such as fast-track designations and orphan drug status are encouraging pharmaceutical companies to invest in antisense technology. This supportive regulatory environment is expected to facilitate the entry of new therapies into the market, potentially increasing the number of approved antisense products. As a result, the Antisense Therapy Market is likely to experience accelerated growth, with projections indicating a market expansion to USD 3.5 billion by 2026.
Rising Investment in Biotechnology
The surge in investment within the biotechnology sector is a significant driver for the Antisense Therapy Market. Venture capital and private equity funding are increasingly directed towards companies specializing in antisense technologies, reflecting a growing confidence in the potential of these therapies. This influx of capital is facilitating research and development efforts, leading to the emergence of novel antisense products. The biotechnology investment landscape is projected to reach USD 50 billion by 2025, with a notable portion allocated to antisense therapy initiatives. This trend underscores the importance of financial backing in advancing the Antisense Therapy Market and fostering innovation.
Increasing Prevalence of Genetic Disorders
The rising incidence of genetic disorders is a pivotal driver for the Antisense Therapy Market. As genetic conditions become more prevalent, the demand for targeted therapies that can address these specific ailments intensifies. For instance, conditions such as Duchenne Muscular Dystrophy and Spinal Muscular Atrophy have garnered significant attention, leading to increased research and development efforts. The market for antisense oligonucleotides is projected to reach approximately USD 3 billion by 2026, reflecting a compound annual growth rate of around 15%. This growth underscores the urgent need for innovative therapies that can effectively target genetic mutations, thereby propelling the Antisense Therapy Market forward.