Increased Research Funding
The surge in research funding for neurological disorders is a notable driver of the aSAH drug market. Government and private organizations are allocating substantial resources to understand the pathophysiology of aSAH and to develop novel therapeutic agents. For instance, funding for clinical trials has seen a marked increase, with millions of dollars being invested annually. This financial support encourages pharmaceutical companies to explore innovative drug formulations and treatment protocols, which could lead to breakthroughs in the management of aSAH. As a result, the aSAH drug market is poised for expansion as new therapies emerge from this robust research environment.
Growing Awareness and Education
There is a growing awareness and education regarding aneurysmal subarachnoid hemorrhage, which is positively impacting the aSAH drug market. Increased public and professional knowledge about the symptoms and risks associated with aSAH is leading to earlier diagnosis and treatment. Educational campaigns by health organizations and advocacy groups are instrumental in disseminating information, which in turn drives demand for effective pharmacological interventions. As more individuals seek medical attention promptly, the need for effective drugs to manage aSAH is likely to rise, thereby fostering growth in the aSAH drug market.
Advancements in Medical Technology
Technological advancements in medical imaging and surgical techniques are significantly influencing the aSAH drug market. Enhanced imaging modalities, such as high-resolution MRI and CT scans, facilitate early diagnosis and intervention, which is crucial for improving patient outcomes. Furthermore, the integration of minimally invasive surgical techniques has transformed the management of aSAH, leading to better recovery rates. These advancements not only improve patient care but also stimulate the demand for new pharmacological agents that can complement these technologies, thereby driving growth in the aSAH drug market.
Regulatory Incentives for Drug Development
Regulatory incentives aimed at expediting drug development are playing a crucial role in shaping the aSAH drug market. Authorities are increasingly offering fast-track designations and priority review processes for drugs targeting critical conditions like aSAH. These incentives not only reduce the time required for new therapies to reach the market but also encourage pharmaceutical companies to invest in the development of innovative treatments. As a result, the aSAH drug market is likely to witness an influx of new products, enhancing treatment options for patients and driving overall market growth.
Rising Incidence of Aneurysmal Subarachnoid Hemorrhage
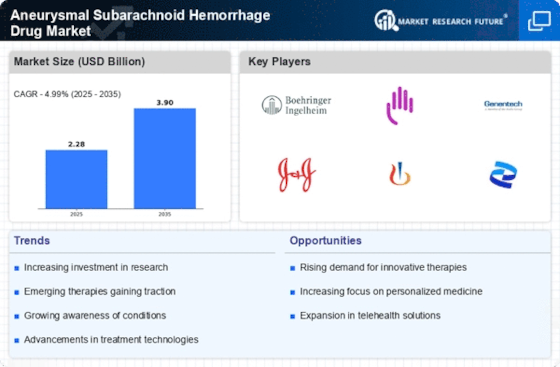
The increasing incidence of aneurysmal subarachnoid hemorrhage (aSAH) is a primary driver for the aSAH drug market. Recent studies indicate that the annual incidence of aSAH ranges from 6 to 16 cases per 100,000 individuals. This rise in cases necessitates the development and availability of effective therapeutic options. As the population ages and risk factors such as hypertension and smoking persist, the demand for innovative treatments is likely to grow. Consequently, pharmaceutical companies are investing in research and development to address this urgent medical need, thereby propelling the aSAH drug market forward.