Growing Awareness and Advocacy
Growing awareness and advocacy for rare diseases, including Andersen Tawil Syndrome, are pivotal in shaping the Andersen Tawil Syndrome Treatment Market Industry. Advocacy groups and patient organizations are playing a crucial role in raising awareness about the syndrome, which in turn drives demand for effective treatments. Increased visibility leads to more individuals seeking diagnosis and treatment, thereby expanding the patient population. Furthermore, advocacy efforts often result in policy changes that favor research funding and support for rare disease initiatives. As more stakeholders become involved in the conversation surrounding Andersen Tawil Syndrome, the market is likely to experience heightened interest from both healthcare providers and pharmaceutical companies. This collective effort may ultimately lead to improved treatment options and better patient outcomes.
Advancements in Genetic Research
Advancements in genetic research are significantly influencing the Andersen Tawil Syndrome Treatment Market Industry. The identification of specific genetic mutations associated with the syndrome has opened new avenues for targeted therapies. As researchers continue to unravel the complexities of the genetic underpinnings of Andersen Tawil Syndrome, the potential for developing personalized treatment options increases. This progress not only enhances the understanding of the disorder but also fosters collaboration among pharmaceutical companies, academic institutions, and research organizations. The market is likely to benefit from the introduction of novel therapies that address the root causes of the syndrome, rather than merely managing symptoms. Consequently, the ongoing research efforts are expected to drive innovation and investment in the treatment landscape.
Regulatory Support for Orphan Drugs
Regulatory support for orphan drugs is a significant factor influencing the Andersen Tawil Syndrome Treatment Market Industry. Governments around the world are implementing policies that incentivize the development of treatments for rare diseases, including Andersen Tawil Syndrome. These incentives often include tax breaks, extended market exclusivity, and expedited approval processes. Such regulatory frameworks encourage pharmaceutical companies to invest in research and development for orphan drugs, which may lead to the introduction of innovative therapies tailored for this specific patient population. As the regulatory landscape continues to evolve, it is likely that more companies will enter the market, enhancing competition and ultimately benefiting patients. This supportive environment is crucial for fostering advancements in treatment options for Andersen Tawil Syndrome.
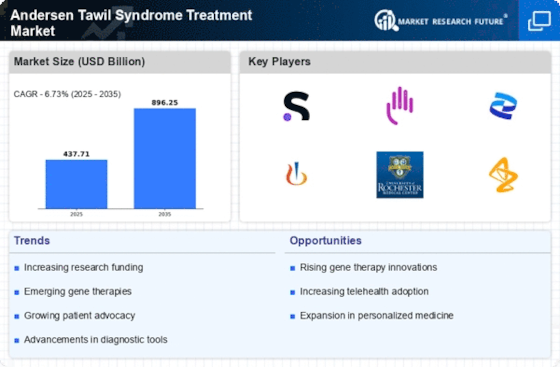
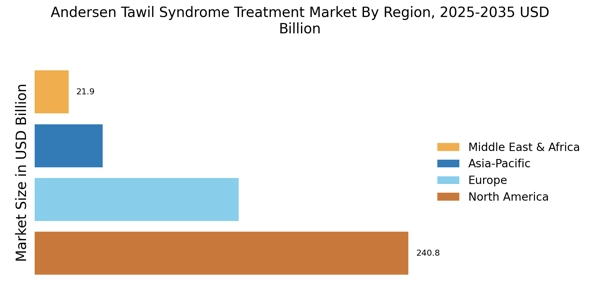
Rising Prevalence of Andersen Tawil Syndrome
The increasing incidence of Andersen Tawil Syndrome is a notable driver for the Andersen Tawil Syndrome Treatment Market Industry. Recent estimates suggest that the prevalence of this rare genetic disorder may be higher than previously understood, with cases reported across various demographics. This rise in prevalence necessitates the development of effective treatment options, thereby stimulating market growth. As awareness of the syndrome expands among healthcare professionals and patients, the demand for specialized treatments is likely to increase. Furthermore, the growing number of diagnosed cases may lead to enhanced funding for research and development, which could further propel the market forward. The urgency to address the needs of affected individuals is becoming more pronounced, indicating a robust market potential for innovative therapies.
Increased Investment in Rare Disease Research
The growing investment in rare disease research is a critical driver for the Andersen Tawil Syndrome Treatment Market Industry. Governments and private organizations are increasingly recognizing the need to allocate resources towards the development of treatments for rare conditions, including Andersen Tawil Syndrome. This trend is reflected in the rising number of grants and funding opportunities aimed at supporting research initiatives. As financial backing increases, researchers are better equipped to explore new therapeutic avenues and clinical trials. The potential for lucrative returns on investment in the rare disease sector is attracting pharmaceutical companies, which may lead to a surge in innovative treatment options. This influx of capital is likely to enhance the overall treatment landscape for Andersen Tawil Syndrome.
















Leave a Comment