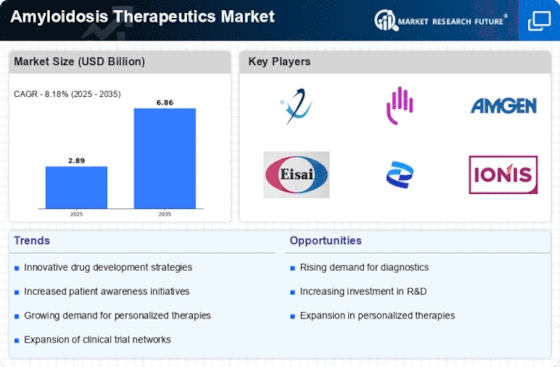
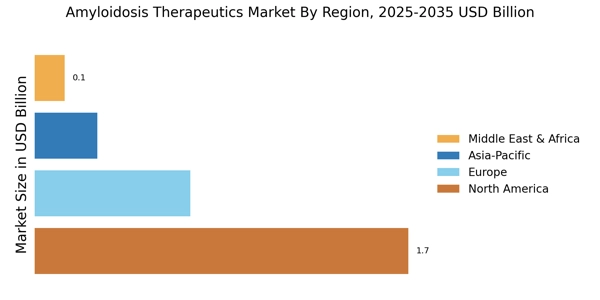
Rising Prevalence of Amyloidosis
The increasing incidence of amyloidosis is a pivotal driver for the Amyloidosis Therapeutics Market. As awareness of this rare disease grows, more cases are being diagnosed, leading to a heightened demand for effective treatment options. Recent estimates suggest that the prevalence of amyloidosis may be underreported, with actual figures potentially being much higher. This rise in diagnosed cases is likely to stimulate the market, as healthcare providers seek innovative therapies to manage the condition. Furthermore, the aging population is contributing to this trend, as older individuals are more susceptible to amyloidosis. Consequently, the need for specialized therapeutics is expected to expand, thereby propelling the Amyloidosis Therapeutics Market forward.
Increased Awareness and Education
The rising awareness and education surrounding amyloidosis are crucial drivers for the Amyloidosis Therapeutics Market. Efforts by healthcare organizations and advocacy groups to educate both the public and medical professionals about the disease are leading to earlier diagnoses and treatment. Increased awareness is likely to result in more patients seeking medical advice, thereby driving demand for therapeutics. Additionally, educational initiatives are fostering collaboration among researchers, clinicians, and pharmaceutical companies, which may lead to innovative treatment solutions. As knowledge about amyloidosis expands, the market is poised for growth, with a broader range of therapeutic options becoming available to patients.
Regulatory Support for New Therapies
Regulatory bodies are increasingly supportive of the development of new therapies for amyloidosis, which is a significant driver for the Amyloidosis Therapeutics Market. Initiatives aimed at expediting the approval process for orphan drugs are encouraging pharmaceutical companies to invest in amyloidosis treatments. This regulatory environment fosters innovation and allows for faster access to potentially life-saving therapies for patients. The introduction of programs such as priority review and accelerated approval is likely to enhance the market landscape, as more drugs enter the pipeline. As a result, the Amyloidosis Therapeutics Market is expected to benefit from a growing array of treatment options, ultimately improving patient outcomes.
Advancements in Diagnostic Techniques
Innovations in diagnostic methodologies are significantly influencing the Amyloidosis Therapeutics Market. Enhanced imaging techniques and biomarker identification have improved the accuracy and speed of amyloidosis diagnosis. This advancement allows for earlier intervention, which is crucial for effective treatment outcomes. As diagnostic capabilities improve, more patients are likely to be identified and treated, thereby increasing the demand for therapeutics. The market is witnessing a shift towards precision medicine, where tailored therapies are developed based on individual patient profiles. This trend not only enhances patient care but also drives the growth of the Amyloidosis Therapeutics Market, as pharmaceutical companies invest in research to develop targeted treatments.
Growing Investment in Biopharmaceuticals
The surge in investment within the biopharmaceutical sector is a key driver for the Amyloidosis Therapeutics Market. As funding for research and development increases, pharmaceutical companies are more inclined to explore novel therapeutic options for amyloidosis. This influx of capital is facilitating the development of innovative drugs, including monoclonal antibodies and gene therapies, which hold promise for treating this complex disease. Market data indicates that the biopharmaceutical industry is experiencing robust growth, with projections suggesting a continued upward trajectory. This financial commitment to research is likely to yield breakthroughs in amyloidosis treatment, thereby expanding the therapeutic landscape and enhancing the Amyloidosis Therapeutics Market.