Innovative Therapeutic Developments
The Acute Repetitive Seizures Market is witnessing a surge in innovative therapeutic developments. Recent advancements in pharmacological treatments, including the introduction of novel antiepileptic drugs, have shown promise in managing acute repetitive seizures effectively. For instance, medications that target specific neurotransmitter systems are being explored, potentially offering better efficacy and fewer side effects. Additionally, the development of personalized medicine approaches, which consider individual patient profiles, is likely to enhance treatment outcomes. This focus on innovation not only addresses the immediate needs of patients but also stimulates competition among pharmaceutical companies, further driving market growth as they strive to bring effective solutions to the forefront.
Regulatory Support for New Treatments
Regulatory support for new treatments is a pivotal factor influencing the Acute Repetitive Seizures Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for innovative therapies that address unmet medical needs. This trend is particularly relevant for acute repetitive seizures, where timely intervention can significantly impact patient outcomes. The introduction of programs aimed at fast-tracking drug development and approval is likely to encourage pharmaceutical companies to invest in research and development. As a result, the market may see a rise in the availability of effective treatments, ultimately benefiting patients and healthcare providers alike.
Growing Demand for Patient-Centric Care
The shift towards patient-centric care is reshaping the Acute Repetitive Seizures Market. Patients are increasingly seeking treatments that not only address their medical needs but also consider their quality of life. This demand is prompting healthcare providers to adopt more holistic approaches, integrating lifestyle modifications and supportive therapies alongside traditional medical treatments. As a result, pharmaceutical companies are likely to develop products that align with this trend, focusing on ease of use and improved patient adherence. This evolution in care delivery is expected to enhance patient satisfaction and outcomes, thereby driving market growth as more individuals seek effective management strategies for acute repetitive seizures.
Increased Investment in Neurological Research
Investment in neurological research is a critical driver for the Acute Repetitive Seizures Market. Governments and private entities are increasingly allocating funds to understand the underlying mechanisms of seizures and develop effective treatments. For instance, research initiatives aimed at exploring the genetic and environmental factors contributing to acute repetitive seizures are gaining traction. This influx of funding is likely to accelerate the pace of discovery, leading to breakthroughs in treatment options. Moreover, collaborations between academic institutions and pharmaceutical companies are fostering an environment conducive to innovation, which may result in the introduction of new therapies and technologies that address the needs of patients suffering from acute repetitive seizures.
Rising Incidence of Acute Repetitive Seizures
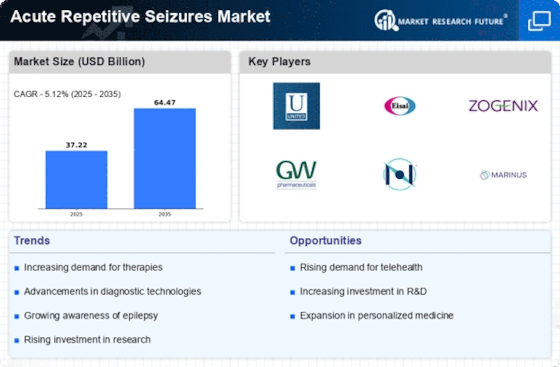
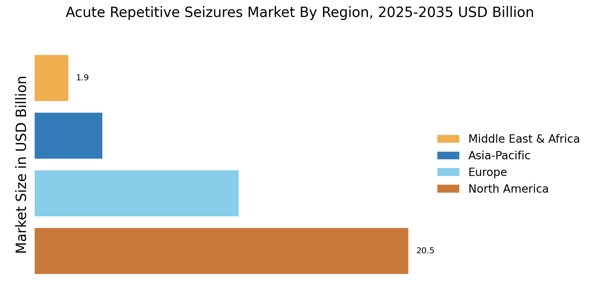
The increasing prevalence of acute repetitive seizures is a notable driver in the Acute Repetitive Seizures Market. Recent estimates suggest that approximately 1 in 26 individuals may develop epilepsy at some point in their lives, with a significant subset experiencing acute repetitive seizures. This rise in incidence necessitates enhanced treatment options and management strategies, thereby propelling market growth. Furthermore, the aging population, which is more susceptible to neurological disorders, contributes to this trend. As the number of patients requiring effective interventions grows, pharmaceutical companies and healthcare providers are likely to invest more in research and development, leading to innovative therapies and solutions tailored for acute repetitive seizures.