Rising Incidence of Acromegaly
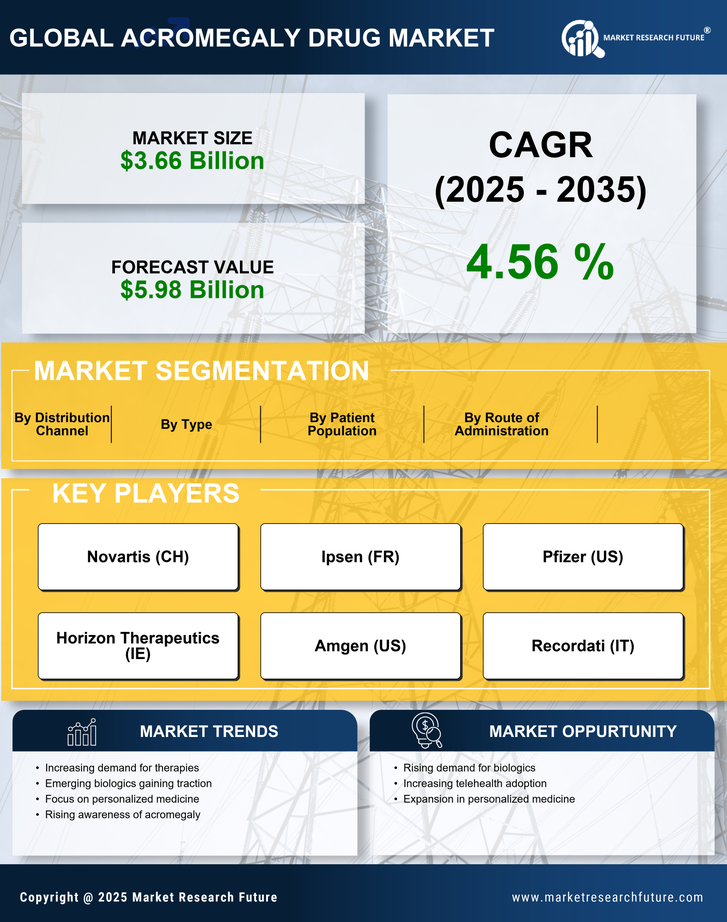
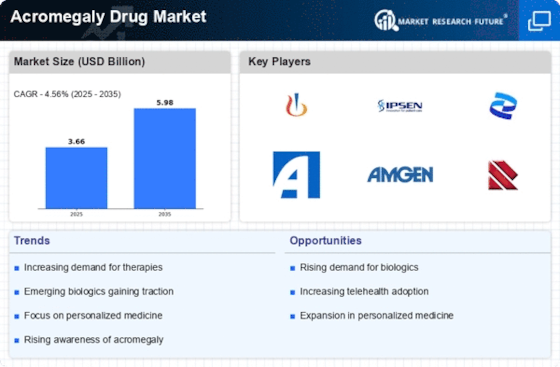
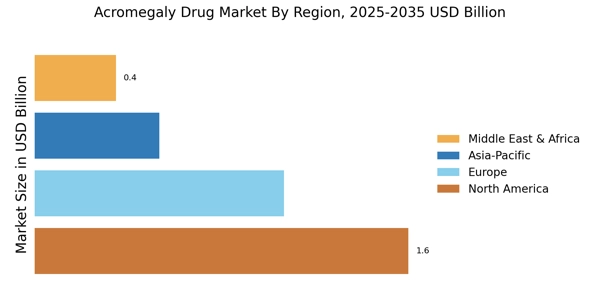
The increasing incidence of acromegaly is a pivotal driver for the Acromegaly Drug Market. Recent estimates suggest that the prevalence of acromegaly ranges from 3 to 13 cases per million people, indicating a substantial patient population requiring treatment. This rise in cases is attributed to improved diagnostic techniques and heightened awareness among healthcare professionals. As more individuals are diagnosed, the demand for effective therapeutic options escalates, thereby propelling the growth of the Acromegaly Drug Market. Furthermore, the aging population, which is more susceptible to hormonal disorders, contributes to this trend. Consequently, pharmaceutical companies are likely to invest in research and development to address the unmet needs of this expanding patient demographic.
Advancements in Treatment Modalities
Innovations in treatment modalities are significantly influencing the Acromegaly Drug Market. The introduction of novel therapies, such as long-acting somatostatin analogs and growth hormone receptor antagonists, has transformed the therapeutic landscape for acromegaly. These advancements not only enhance treatment efficacy but also improve patient compliance due to reduced dosing frequency. Market data indicates that the somatostatin analog segment is projected to witness substantial growth, driven by its effectiveness in controlling growth hormone levels. Additionally, the development of combination therapies may further optimize treatment outcomes, thereby attracting more patients to seek medical intervention. As a result, the Acromegaly Drug Market is poised for expansion, with ongoing research likely to yield even more innovative solutions.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy and support groups is playing a vital role in shaping the Acromegaly Drug Market. These organizations are instrumental in raising awareness about acromegaly, educating patients about their treatment options, and advocating for better healthcare policies. Their efforts contribute to increased patient engagement and empowerment, which can lead to higher treatment adherence rates. Furthermore, these groups often collaborate with pharmaceutical companies to facilitate clinical trials and gather real-world evidence on treatment effectiveness. As patient advocacy continues to grow, it is likely to influence the demand for new therapies and drive the development of patient-centered solutions within the Acromegaly Drug Market. This dynamic interaction between patients and industry stakeholders may foster a more responsive healthcare environment.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a significant driver of the Acromegaly Drug Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for drugs targeting rare diseases, including acromegaly. Initiatives such as breakthrough therapy designations and fast track designations are designed to facilitate the development and availability of new treatments. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that their innovative solutions may reach the market more swiftly. As a result, the Acromegaly Drug Market is likely to experience a surge in new product launches, enhancing treatment options for patients. The interplay between regulatory frameworks and industry innovation is expected to create a more vibrant market landscape.
Increased Investment in Rare Disease Research
The growing investment in research for rare diseases, including acromegaly, is a crucial driver for the Acromegaly Drug Market. Pharmaceutical companies and research institutions are increasingly allocating resources to develop targeted therapies for conditions that have historically been overlooked. This trend is partly fueled by regulatory incentives, such as orphan drug designations, which provide financial benefits and market exclusivity for developers. As a result, the number of clinical trials focusing on acromegaly is on the rise, leading to a broader array of treatment options. Market analysts project that this influx of investment will not only enhance the therapeutic landscape but also stimulate competition among manufacturers, ultimately benefiting patients with acromegaly. The Acromegaly Drug Market stands to gain from these developments, as new entrants and innovative products emerge.