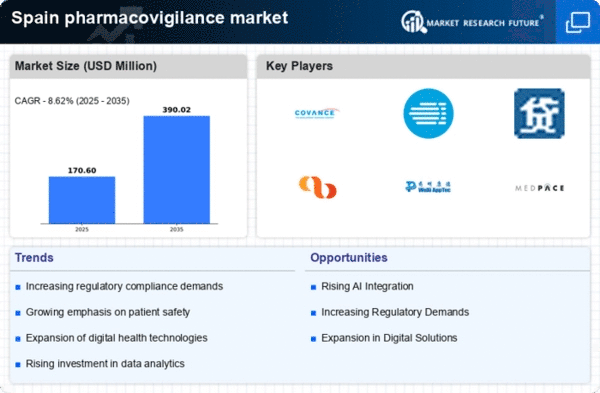
The Spain Pharmacovigilance Market is a dynamic and evolving sector characterized by a complex interplay of regulatory requirements, technological advancements, and the imperative for patient safety.
The competitive landscape is shaped by various factors including the increasing focus on drug safety monitoring, the rise in adverse drug reaction reporting, and the necessity for compliance with stringent regulations set forth by both national and European legislative bodies.
Several key players are vying for market share, each leveraging unique capabilities to capitalize on this growing demand. With an emphasis on innovative solutions, the landscape is becoming increasingly competitive, characterized by strategic partnerships, collaborations, and investments aimed at enhancing pharmacovigilance processes.
Oracle stands out in the Spain Pharmacovigilance Market due to its comprehensive suite of software solutions designed to streamline drug safety management and regulatory compliance. The company enjoys a strong market presence facilitated by its robust technological infrastructure and an unparalleled reputation for reliability.
Oracle’s strength lies in its ability to provide integrated solutions that cater to the diverse needs of pharmaceutical companies, enabling them to efficiently collect, process, and analyze safety data. This is bolstered by a dedicated local presence that allows for tailored customer service and support, ensuring that clients in Spain are equipped to meet stringent pharmacovigilance requirements.
Furthermore, Oracle's continuous investment in research and development enables it to stay ahead of regulatory changes and market demands, solidifying its competitive edge. Medpace is notable in the Spain Pharmacovigilance Market as a full-service contract research organization that offers a diverse range of services, including drug development, clinical trial management, and safety reporting.
The company's strong presence in Spain is highlighted by its commitment to providing high-quality clinical research services that comply with both local and international standards. Medpace's strengths lie in its experienced team who specialize in pharmacovigilance, providing thorough monitoring and reporting services that enhance the safety profiles of pharmaceuticals.
The organization has pursued strategic mergers and acquisitions, allowing it to expand its capabilities and enhance service offerings in the Spanish market.
Medpace's comprehensive understanding of the regulatory landscape, coupled with its capability to deliver end-to-end solutions, enables it to effectively address the unique challenges faced by stakeholders in Spain, ultimately supporting the safety of drug therapies in the region.