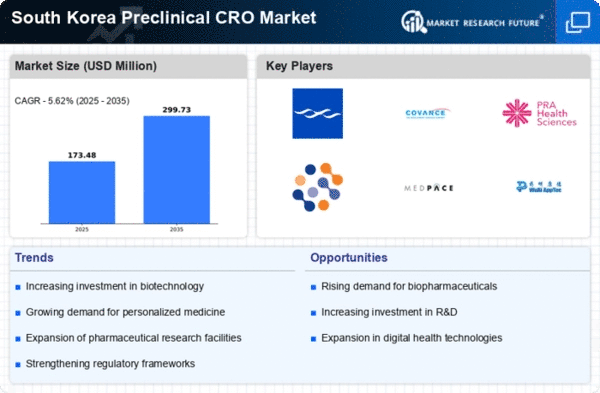
South Korea Preclinical CRO Market Summary
The South Korea Preclinical CRO market is projected to grow from 164.2 USD Million in 2024 to 400 USD Million by 2035, reflecting a robust growth trajectory.
Key Market Trends & Highlights
South Korea Preclinical CRO Key Trends and Highlights
- The market is expected to achieve a compound annual growth rate (CAGR) of 8.43% from 2025 to 2035.
- By 2035, the market valuation is anticipated to reach 400 USD Million, indicating substantial growth opportunities.
- In 2024, the market is valued at 164.2 USD Million, showcasing a solid foundation for future expansion.
- Growing adoption of innovative drug development technologies due to increasing investment in research and development is a major market driver.
Market Size & Forecast
| 2024 Market Size | 164.2 (USD Million) |
| 2035 Market Size | 400 (USD Million) |
| CAGR (2025-2035) | 8.43% |
Major Players
SillaJen, Medytox, Samsung Biologics, Korea Biomedicine Industry Association, Korea Research Institute of Chemical Technology, Inovio Pharmaceuticals, Hanmi Pharmaceutical, GENNBIO, Institute of Drug Development, LG Chem, Severance Hospital, Celltrion, Daewoong Pharmaceutical