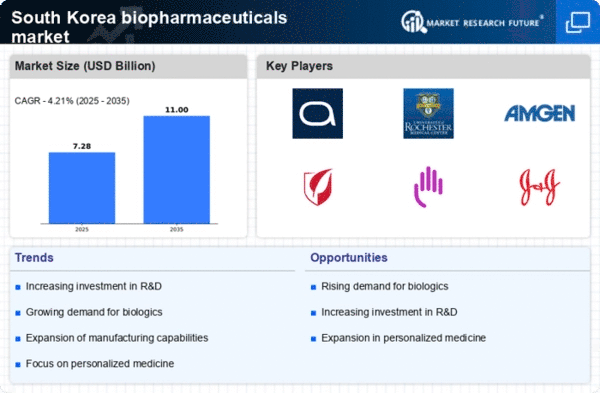
A number of variables, such as increased healthcare demands, government backing, and technical improvements, are driving key trends in the South Korean biopharmaceuticals market. Through its "Biopharma Vision 2025" project, the South Korean government has aggressively encouraged biopharmaceutical research with the goal of making the nation a premier biotechnology powerhouse.
By improving research and development efforts, this assistance fosters an atmosphere that supports the expansion of biopharmaceutical businesses. Additionally, the need for improved treatments is being driven by the ageing population and the rising prevalence of chronic diseases, which encourages businesses to concentrate on developing new drugs.
Furthermore, there are a lot of market potential to investigate, especially in the areas of biosimilars and personalised medicine. South Korean patient care may change as a result of local businesses creating customised treatments that are tailored to each patient's unique profile in response to the growing popularity of targeted therapies. Furthermore, because of their well-established clinical research capabilities and manufacturing procedures, South Korean companies are well-positioned to benefit from the growing global biosimilars industry.
Additionally, the government supports industry-academia collaborations, which promote creativity and quicken the creation of novel biopharmaceutical products. Collaborations and partnerships between South Korean research institutes, universities, and biopharmaceutical companies have significantly increased in recent years.
These partnerships are crucial for exchanging information, assets, and technology, all of which can hasten the development of novel medicinal products. Additionally, the emergence of digital health technologies, such as big data and artificial intelligence, is changing the way that pharmaceuticals are created, assessed, and marketed in South Korea as well as the way that biopharmaceutical research is carried out. In order to improve clinical trial efficiency and streamline processes, this digital transition is becoming essential. Strong governmental regulations and rising healthcare demands are driving the biopharmaceuticals market in South Korea to grow and innovate dynamically overall.