Market Growth Projections
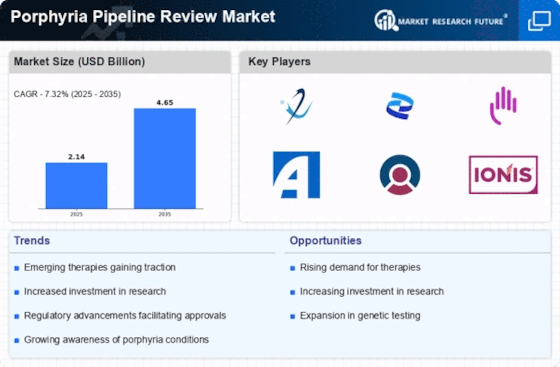
The Global Porphyria Pipeline Review Market Industry exhibits promising growth projections, with an anticipated market value of 2.14 USD Billion in 2024, expected to escalate to 4.65 USD Billion by 2035. This trajectory reflects a compound annual growth rate (CAGR) of 7.3% from 2025 to 2035, indicating a robust pipeline of therapeutic candidates. The increasing focus on research and development, coupled with supportive regulatory frameworks, is likely to drive this growth. As the market evolves, stakeholders are expected to invest in innovative solutions to address the unmet needs of porphyria patients, further enhancing the industry's landscape.
Growing Awareness and Education
Heightened awareness and educational initiatives surrounding porphyria are crucial drivers for the Global Porphyria Pipeline Review Market Industry. Increased understanding among healthcare professionals and patients leads to earlier diagnosis and treatment, which is essential for managing this complex group of disorders. As awareness campaigns proliferate, the market is expected to see a surge in demand for effective therapies. This trend aligns with the projected market growth, with an estimated value of 4.65 USD Billion by 2035. The emphasis on education may also foster collaboration among researchers and clinicians, further advancing the development of new treatment options.
Advancements in Drug Development
Innovations in biotechnology and pharmacology are propelling the Global Porphyria Pipeline Review Market Industry forward. The emergence of novel therapeutic agents, including gene therapies and biologics, is expected to enhance treatment efficacy and safety profiles. As of 2024, the market is valued at 2.14 USD Billion, with projections suggesting it could reach 4.65 USD Billion by 2035. This growth is indicative of a robust pipeline, with numerous candidates undergoing clinical trials. The focus on personalized medicine may also play a crucial role in tailoring treatments to individual patient needs, thereby improving outcomes and driving market growth.
Regulatory Support and Incentives
Government initiatives and regulatory frameworks are increasingly supportive of drug development for rare diseases, including porphyria. The Global Porphyria Pipeline Review Market Industry benefits from policies that incentivize research and development, such as orphan drug designations and fast-track approvals. These measures are designed to expedite the availability of new therapies to patients in need. As the market evolves, the anticipated compound annual growth rate (CAGR) of 7.3% from 2025 to 2035 suggests a favorable environment for investment and innovation. This regulatory landscape is likely to attract more stakeholders, enhancing the overall market dynamics.
Increasing Prevalence of Porphyria
The rising incidence of porphyria globally is a primary driver for the Global Porphyria Pipeline Review Market Industry. As awareness of this rare group of disorders grows, more cases are being diagnosed, leading to an increased demand for effective treatments. In 2024, the market is projected to reach 2.14 USD Billion, reflecting the urgent need for innovative therapies. The growing patient population necessitates advancements in drug development, which is expected to contribute to the market's expansion. This trend indicates a potential for significant growth in the coming years as healthcare systems adapt to manage these complex conditions.
Emerging Markets and Global Expansion
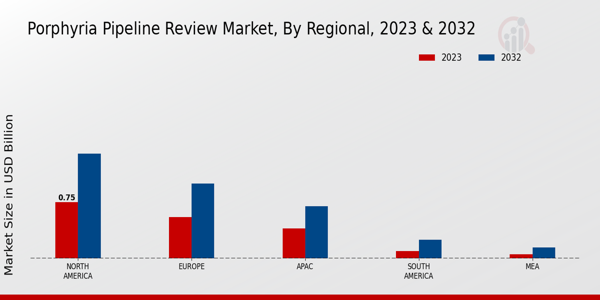
The Global Porphyria Pipeline Review Market Industry is witnessing growth in emerging markets, where healthcare infrastructure is improving. Countries in Asia and Latin America are increasingly investing in healthcare, leading to enhanced access to diagnostic and therapeutic options for porphyria patients. This expansion is expected to contribute to the market's growth trajectory, with the overall market projected to reach 4.65 USD Billion by 2035. The increasing number of healthcare facilities and trained professionals in these regions may facilitate better management of porphyria, thereby driving demand for innovative treatments. This trend highlights the potential for global collaboration in addressing rare diseases.