Market Growth Projections
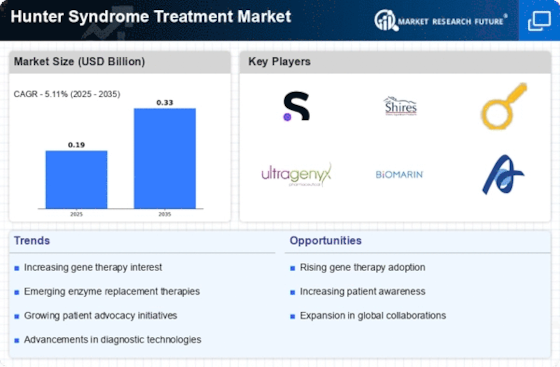
The Global Hunter Syndrome Treatment Market Industry is projected to experience substantial growth over the coming years. With an estimated market value of 0.19 USD Billion in 2024, it is expected to reach 0.33 USD Billion by 2035. This growth reflects a compound annual growth rate (CAGR) of 5.01% from 2025 to 2035, indicating a steady increase in demand for effective treatments. The market dynamics are influenced by various factors, including advancements in therapy, increased awareness, and regulatory support. These projections highlight the potential for continued development and investment in the treatment of Hunter Syndrome.
Increased Research Funding
The Global Hunter Syndrome Treatment Market Industry is bolstered by increased funding for research and development initiatives. Government and private organizations are prioritizing rare diseases, leading to enhanced investment in Hunter Syndrome research. This influx of funding supports clinical trials, which are essential for the development of new therapies. As a result, the market is likely to benefit from innovative treatment options emerging from these research efforts. The projected compound annual growth rate (CAGR) of 5.01% from 2025 to 2035 indicates a promising future for the industry as new therapies are developed and brought to market.
Growing Awareness and Advocacy
Growing awareness and advocacy for Hunter Syndrome play a crucial role in shaping the Global Hunter Syndrome Treatment Market Industry. Patient advocacy groups and healthcare organizations are actively working to educate the public and healthcare professionals about the disorder. This increased awareness leads to earlier diagnosis and treatment, ultimately improving patient outcomes. As more individuals become informed about Hunter Syndrome, the demand for effective treatments is expected to rise. This trend is likely to contribute to the market's growth trajectory, as stakeholders recognize the importance of addressing the needs of affected individuals.
Rising Prevalence of Hunter Syndrome
The Global Hunter Syndrome Treatment Market Industry is experiencing growth due to the increasing prevalence of Hunter Syndrome, a rare genetic disorder. As awareness of this condition rises, more cases are being diagnosed. Current estimates suggest that Hunter Syndrome affects approximately 1 in 162,000 births globally. This growing patient population necessitates the development of effective treatment options, thereby driving market demand. In 2024, the market is projected to reach 0.19 USD Billion, reflecting the urgent need for innovative therapies and management strategies to address the complexities of this disorder.
Advancements in Enzyme Replacement Therapy
Recent advancements in enzyme replacement therapy (ERT) have significantly impacted the Global Hunter Syndrome Treatment Market Industry. ERT has emerged as a cornerstone of treatment, providing patients with the missing enzyme necessary for metabolic function. Innovative therapies, such as idursulfase, have demonstrated efficacy in improving clinical outcomes. As these therapies gain regulatory approval and become more widely available, they are expected to enhance patient quality of life. The market is anticipated to grow to 0.33 USD Billion by 2035, driven by the ongoing development of novel ERT options that cater to diverse patient needs.
Regulatory Support for Rare Disease Treatments
Regulatory support for rare disease treatments is a significant driver of the Global Hunter Syndrome Treatment Market Industry. Governments worldwide are implementing policies that facilitate the development and approval of therapies for rare conditions. Initiatives such as orphan drug designations and expedited review processes encourage pharmaceutical companies to invest in Hunter Syndrome treatments. This supportive regulatory environment is likely to accelerate the introduction of new therapies, ultimately benefiting patients. As the market evolves, the combination of regulatory incentives and innovative treatment options may lead to a more robust industry landscape.