Market Trends and Projections
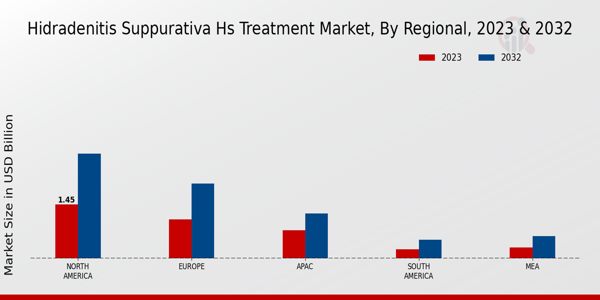
The Global Hidradenitis Suppurativa HS Treatment Market Industry is projected to maintain a steady trajectory, with a market value of 3.77 USD Billion in 2024 and remaining stable through 2035. The compound annual growth rate (CAGR) is anticipated to be 0.0% for the period between 2025 and 2035. This stability may reflect a maturation of the market, where existing treatment options become well-established, and new innovations emerge at a slower pace. Monitoring these trends will be essential for stakeholders aiming to navigate the evolving landscape of HS treatment.
Supportive Government Policies
Supportive government policies and initiatives aimed at improving healthcare access and treatment options for chronic conditions like Hidradenitis Suppurativa play a vital role in the Global Hidradenitis Suppurativa HS Treatment Market Industry. Governments are increasingly recognizing the need for comprehensive care models that include funding for research and development of new therapies. Such policies not only enhance patient access to treatments but also encourage pharmaceutical companies to invest in HS research. This supportive environment is likely to maintain the market's projected value of 3.77 USD Billion through 2035, fostering innovation and improving patient outcomes.
Increased Awareness and Diagnosis
Heightened awareness of Hidradenitis Suppurativa among healthcare professionals and the general public contributes to the growth of the Global Hidradenitis Suppurativa HS Treatment Market Industry. Improved diagnostic criteria and educational initiatives have led to earlier detection and treatment of HS, which is crucial for managing the condition effectively. As more patients receive timely diagnoses, the demand for treatment options rises. This trend is expected to sustain the market's value at 3.77 USD Billion in 2024, as healthcare systems adapt to accommodate the increasing number of diagnosed cases.
Advancements in Treatment Modalities
Innovations in treatment options significantly influence the Global Hidradenitis Suppurativa HS Treatment Market Industry. The introduction of biologics and targeted therapies has transformed the management landscape for HS, offering patients more effective and personalized treatment regimens. For instance, medications such as adalimumab have shown promising results in clinical trials, leading to improved patient outcomes. As these advancements continue, they are likely to attract investment and research, further enhancing the market's growth potential. The market is expected to maintain a value of 3.77 USD Billion by 2035, reflecting the ongoing evolution of treatment strategies.
Emerging Markets and Economic Growth
The expansion of healthcare infrastructure in emerging markets contributes to the growth of the Global Hidradenitis Suppurativa HS Treatment Market Industry. As economies develop, there is an increasing focus on improving healthcare access and quality, which includes the availability of treatments for chronic conditions like HS. Countries in Asia-Pacific and Latin America are witnessing significant investments in healthcare, leading to enhanced treatment options for HS patients. This trend may support a steady market value of 3.77 USD Billion by 2035, as these regions become more integrated into the global healthcare landscape.
Rising Prevalence of Hidradenitis Suppurativa
The increasing incidence of Hidradenitis Suppurativa (HS) globally drives the Global Hidradenitis Suppurativa HS Treatment Market Industry. Recent estimates suggest that HS affects approximately 1-4% of the population, with higher prevalence noted in specific demographics. This growing patient population necessitates the development and availability of effective treatment options. As awareness of HS improves, more individuals seek medical intervention, thereby expanding the market. The projected market value of 3.77 USD Billion in 2024 reflects this trend, indicating a sustained demand for innovative therapies and management strategies.