- Q1 2024: Otonomy Announces FDA Clearance of IND Application for OTO-313 in Treatment of Vertigo Otonomy received FDA clearance for its Investigational New Drug (IND) application to begin clinical trials of OTO-313, a novel treatment for vertigo, marking a significant regulatory milestone for the company.
- Q2 2024: Hearing Health Company GN Store Nord Acquires Vertigo Diagnostics Startup Vestibule Medical GN Store Nord announced the acquisition of Vestibule Medical, a startup specializing in diagnostic devices for vertigo, expanding GN’s portfolio in the vestibular disorder treatment sector.
- Q2 2024: Otologic Pharmaceuticals Launches VertiCalm, a New OTC Medication for Benign Paroxysmal Positional Vertigo Otologic Pharmaceuticals introduced VertiCalm, an over-the-counter medication targeting symptoms of BPPV, aiming to provide accessible relief for vertigo patients.
- Q3 2024: Balance Therapeutics Secures $30 Million Series B Funding to Advance Vertigo Drug Pipeline Balance Therapeutics closed a $30 million Series B funding round to accelerate development of its pipeline of drugs for vertigo and other vestibular disorders.
- Q3 2024: FDA Approves SENS-111 for Acute Vertigo Attacks The FDA granted approval to SENS-111, a selective antihistamine developed for the treatment of acute vertigo attacks, representing a new therapeutic option for patients.
- Q4 2024: Medtronic Opens Dedicated Vertigo Treatment Market Research Facility in Minnesota Medtronic inaugurated a new research facility focused on vertigo treatment technologies, aiming to accelerate innovation in diagnostic and therapeutic solutions for vestibular disorders.
- Q4 2024: VertiTech Partners with Mayo Clinic to Develop AI-Based Vertigo Diagnostic Platform VertiTech announced a strategic partnership with Mayo Clinic to co-develop an artificial intelligence-powered platform for rapid and accurate vertigo diagnosis.
- Q1 2025: Otonomy Appoints Dr. Lisa Chen as Chief Medical Officer to Lead Vertigo Clinical Programs Otonomy named Dr. Lisa Chen as Chief Medical Officer, tasking her with oversight of the company’s clinical programs targeting vertigo and other inner ear disorders.
- Q1 2025: Sanofi Signs Licensing Agreement with Balance Therapeutics for Vertigo Drug Candidate Sanofi entered into a licensing agreement with Balance Therapeutics to co-develop and commercialize a promising vertigo drug candidate, expanding Sanofi’s neurology portfolio.
- Q2 2025: Otologic Pharmaceuticals Receives CE Mark for VertiCalm in Europe Otologic Pharmaceuticals obtained CE Mark approval for VertiCalm, allowing the company to market its vertigo treatment product across the European Union.
- Q2 2025: VertiTech Raises $15 Million in Series A Funding to Expand Vertigo Diagnostic Solutions VertiTech secured $15 million in Series A funding to scale up development and deployment of its vertigo diagnostic technologies in hospitals and clinics.
- Q3 2025: FDA Grants Breakthrough Device Designation to Mayo Clinic’s AI Vertigo Diagnostic Tool The FDA awarded Breakthrough Device Designation to Mayo Clinic’s AI-powered vertigo diagnostic tool, expediting its path to market and clinical adoption.
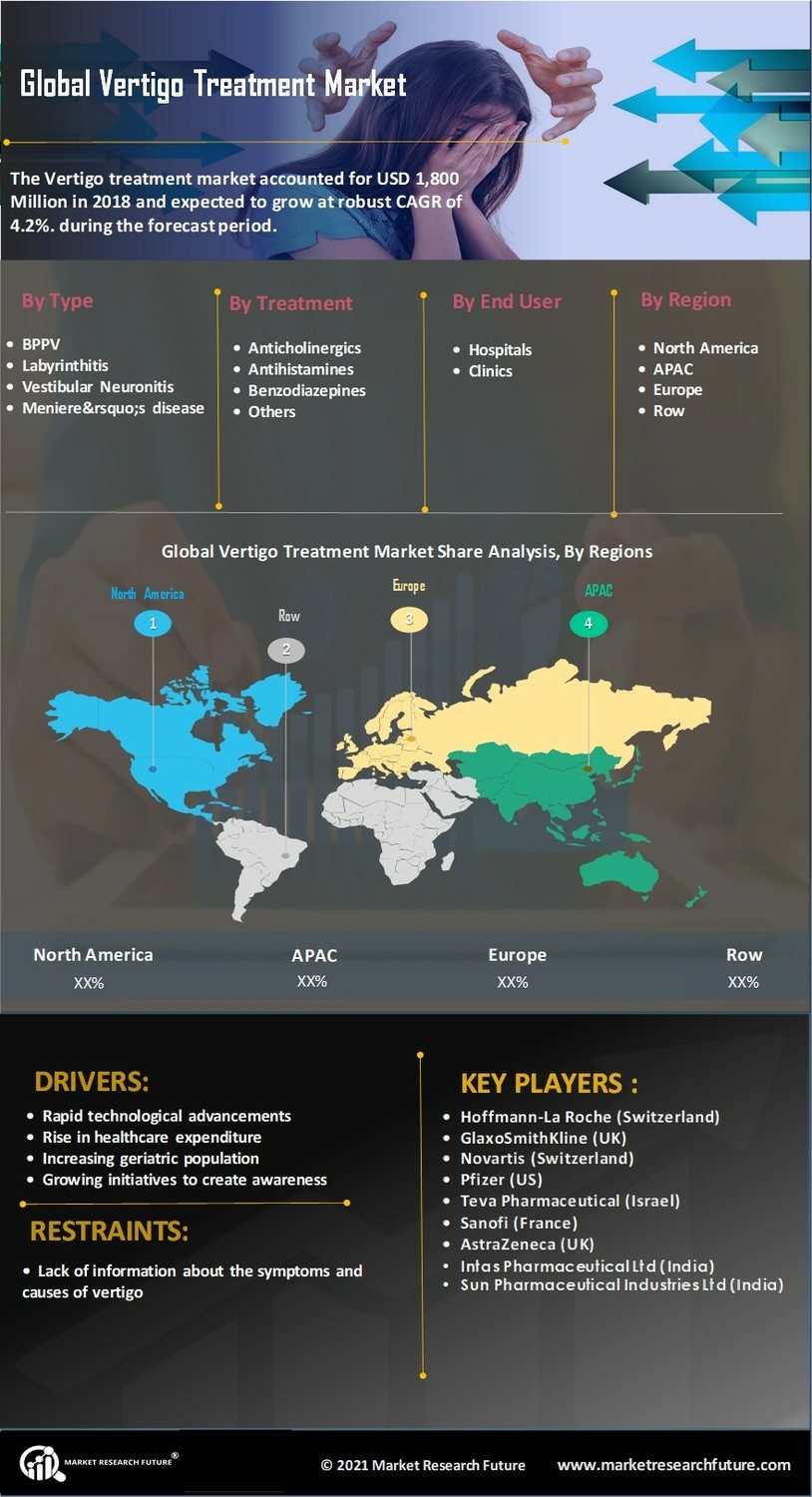
Vertigo Treatment Market Drivers
- Increase in prevalence of vertigo cases
- Cases of vertigo are on the rise, with over 20% 50% of the global population affected by vertigo and According to the National Center for Biotechnology Information, in the US, around 26 million people required a visit to an emergency department for dizziness/vertigo.
- Rapid technological advancements
- Rise in healthcare expenditure
- Increasing geriatric population
- Increasing mergers & acquisitions by prominent market players in the vertigo treatment
- Growing initiatives to create awareness
- For instance, the German Center for Vertigo and Balance Disorders (DSGZ) started an initiative called DIZZYNET. The main aim is to create a platform for collaboration and exchange among scientists, physicians, and physiotherapists to understand the treatment options and epidemiology of vertigo. Such initiates help to create awareness regarding symptoms and treatment for vertigo.
Vertigo Treatment Market Restraints
- Lack of information about the symptoms and causes of vertigo
Vertigo Treatment Market Segment Insights
Vertigo Treatment Market Type Insights
Benign Paroxysmal Positional Vertigo (BPPV): Expected to hold the largest share, as benign paroxysmal positional vertigo is the most common form of peripheral vertigo. This type tends to cause short, frequent bouts of vertigo.
Labyrinthitis: An inner ear infection causes labyrinthitis form of vertigo. This form of vertigo is usually overlooked as they appear with other symptoms such as fever and earache.
Vestibular Neuronitis: Vestibular neuritis is a disorder that affects the nerve of the inner ear. This type of vertigo has a sudden onset and may cause unsteadiness, earache, nausea, and vomiting.
Meniere’s disease: This type of vertigo is often so severe that it causes nausea and vomiting. Meniere’s disease also causes hearing loss, ringing in your ears, and a feeling of fullness in your ears.
Vertigo Treatment Market Treatment Insights
Anticholinergics: Anticholinergics are vestibular suppressants and are most important in treating vertigo since anticholinergic drugs that do not cross the blood-brain barrier are ineffective in controlling motion sickness. The most effective single anticholinergic drug for the prophylaxis and treatment of motion sickness is scopolamine. All anticholinergics conventionally used in the management of vertigo or motion sickness have prominent side-effects, often including dry mouth, dilated pupils, and sedation. Anticholinergics are ineffective if administered after symptoms have already appeared.
Antihistamines: Centrally acting antihistamines prevent motion sickness and reduce the severity of symptoms, even if taken after the onset of the symptoms. All the antihistamines in general use for control of vertigo also have anticholinergic activity.
Benzodiazepines: Benzodiazepines are extremely useful for the management of acute vertigo. They are also helpful in controlling motion sickness and can also minimize anxiety and panic associated with vertigo. Diazepam, clonazepam, lorazepam, and alprazolam are commonly prescribed benzodiazepines for their effect as anxiolytics and antidepressants.
Others: The other segment includes antibiotics and other drugs that can help ease the symptoms of vertigo.
Vertigo Treatment Market End-User Insights
Hospital: Hospitals dominate the global market for vertigo treatment. The positive growth of hospitals is attributed to their rising need to offer treatments for various health disorders. Additionally, the large number of hospitals is also driving the growth for the hospital's segment.
Clinics: Clinics take up the second-largest share due to the rising number of clinics and the increasing patient population.
Vertigo Treatment Market Regional Insights
Americas:It is the largest regional vertigo treatment market owing to the large patient population, rise in prevalence of vertigo, and surge in awareness about the causes of vertigo and its treatments. According to the American Speech-Language-Hearing Association, around 40% of the population in the US experience some form of dizziness or balance difficulty over the course of a lifetime.
Europe:The growing presence of the developed pharmaceutical industry and the significant demand for vertigo related therapeutics is driving the market growth of the vertigo treatment in Europe. According to the National Center for Biotechnology Information, the prevalence of vertigo in Germany was 22.9%.
Asia-Pacific:The Asia-Pacific region is anticipated to be the fastest-growing region in the global market of vertigo treatment. This region has witnessed a surge in the number of people afflicted with vertigo and other neurological disorders.
Middle East & Africa:The smallest market due to limited healthcare infrastructure and limited exposure to technological advancements.
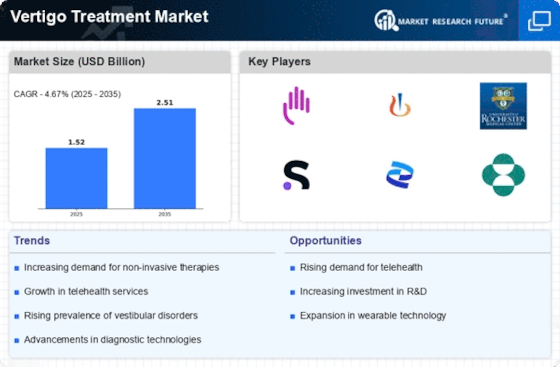
Vertigo Treatment Market Key Players