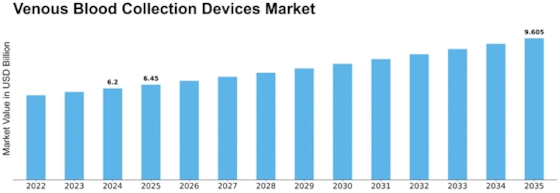
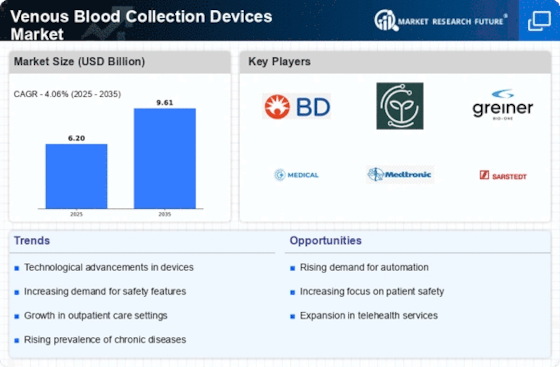
Venous Blood Collection Devices Size
Venous Blood Collection Devices Market Growth Projections and Opportunities
As more medical tests need venous blood samples, the market for venous blood collection devices is expanding. As health care organizations prioritize early illness detection and preventive treatment, dependable and efficient blood collection technologies are needed. This shift alters the market, and firms are developing new devices to fulfill healthcare professionals' demands.
Blood collection equipment' technological advancements impact the market. New technologies include vacuum technology, safety features, and easy-to-use designs make venous blood collection safer and more efficient. Research and development to improve gadgets will give companies an edge. Due to chronic conditions including diabetes, heart disease, and viral disorders, regular blood testing are more vital than ever. Monitoring and diagnosing these disorders requires vein-drawing devices. More individuals having chronic conditions affects the market and boosts blood collection gadget demand. Point-of-care testing and home healthcare have changed the VENOUS BLOOD COLLECTION DEVICES market. People desire portable, easy-to-use blood collection kits for homes and small workplaces as more medical testing are done outside of hospitals and clinics. The market may develop if companies adapt their products to these trends. Regulations heavily impact the venous blood collection devices industry. Market participants must observe stringent quality and legal norms. Following guidelines makes entering a market simpler and develops confidence among healthcare specialists and end customers. Healthcare staff must be adept in phlebotomy to collect venous blood. Companies that invest in healthcare worker training and education affect the market. One sensible strategy to handle this market is to partner with healthcare and educational institutions. Venous blood collection devices have a global market. Opportunities are rising in developing nations. Globalization and healthcare system development in developing nations boost market growth. When going global, corporations must consider how other nations' healthcare systems function and prepare accordingly. Cost cutbacks are exerting pressure on healthcare systems worldwide, affecting medical goods purchases. VENOUS BLOOD COLLECTION DEVICES businesses are developing low-cost, high-quality solutions. Market success requires budget-friendly strategies that maintain product efficacy. Improving patient comfort during blood collection influences the market. Device businesses that alleviate patient pain, suffering, and concern improve patient experiences. This user-focused approach may increase blood collection equipment utilization. Companies form successful collaborations, mergers, and acquisitions in the VENOUS BLOOD COLLECTION DEVICES industry. These alliances aid research and development, product expansion, and market stability. Companies that wish to remain ahead in this shifting industry must monitor their competition.

















Leave a Comment