Regulatory Support and Guidelines
The establishment of stringent regulatory frameworks and guidelines for infection management plays a pivotal role in shaping the Global US Staphylococcus aureus Infection Diagnosis Treatment for Orthopedic Implant Market Industry. Regulatory bodies are increasingly focusing on ensuring the safety and efficacy of diagnostic and therapeutic products. This regulatory support fosters innovation and encourages manufacturers to develop advanced solutions for infection diagnosis and treatment. As the market adapts to these evolving regulations, it is likely to witness sustained growth, with a projected market value of 2750 USD Million by 2035, underscoring the importance of compliance in driving industry advancements.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic methods significantly influence the Global US Staphylococcus aureus Infection Diagnosis Treatment for Orthopedic Implant Market Industry. Enhanced diagnostic tools, such as rapid molecular assays and advanced imaging techniques, facilitate early detection of infections, which is crucial for effective treatment. These advancements not only improve patient outcomes but also reduce the overall burden on healthcare systems. As the market evolves, the integration of these technologies is expected to contribute to a projected market growth, with estimates indicating a rise to 2750 USD Million by 2035, highlighting the importance of timely diagnosis in managing infections.
Growing Awareness and Preventive Measures
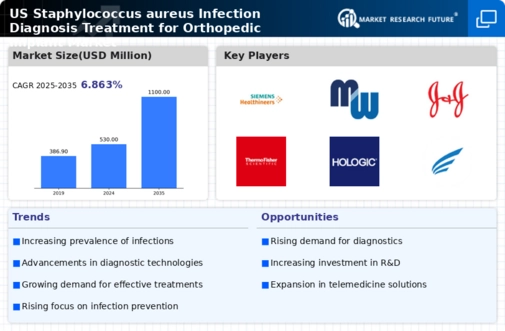
Increased awareness regarding the risks associated with Staphylococcus aureus infections has led to a greater emphasis on preventive measures within the Global US Staphylococcus aureus Infection Diagnosis Treatment for Orthopedic Implant Market Industry. Healthcare professionals are now more vigilant in implementing infection control protocols, which include preoperative screening and postoperative monitoring. This proactive approach not only helps in reducing infection rates but also enhances the overall quality of care. As a result, the market is expected to experience a compound annual growth rate (CAGR) of 7.43% from 2025 to 2035, reflecting the ongoing commitment to improving patient safety.
Increasing Investment in Healthcare Infrastructure
The rising investment in healthcare infrastructure, particularly in surgical facilities, significantly impacts the Global US Staphylococcus aureus Infection Diagnosis Treatment for Orthopedic Implant Market Industry. Enhanced infrastructure enables the adoption of advanced diagnostic and treatment technologies, thereby improving patient care. As healthcare systems expand and modernize, the capacity to manage Staphylococcus aureus infections effectively increases. This trend is expected to contribute to the market's growth trajectory, with a valuation of 1250 USD Million in 2024 and a forecasted increase to 2750 USD Million by 2035, reflecting the ongoing commitment to improving healthcare delivery.
Rising Incidence of Staphylococcus aureus Infections
The increasing prevalence of Staphylococcus aureus infections, particularly in patients with orthopedic implants, drives the Global US Staphylococcus aureus Infection Diagnosis Treatment for Orthopedic Implant Market Industry. As the population ages and the number of orthopedic surgeries rises, the incidence of these infections is expected to grow. In 2024, the market is valued at 1250 USD Million, reflecting the urgent need for effective diagnostic and treatment solutions. This trend is likely to continue, as healthcare providers seek to mitigate the risks associated with infections, thereby enhancing patient outcomes and reducing healthcare costs.
















Leave a Comment