Increased Focus on Quality Control
Quality control remains a paramount concern in the pharmaceutical industry, significantly impacting the pharmaceutical isolator market. The need for high-quality products necessitates the implementation of advanced isolator systems that minimize contamination risks. Regulatory bodies in the US have established stringent guidelines that require manufacturers to adopt isolator technology to ensure product integrity. This focus on quality is reflected in the market, which is expected to reach a valuation of $1.5 billion by 2027. As companies strive to meet these quality standards, investments in isolator technology are likely to increase, driving market growth. The emphasis on quality assurance not only enhances consumer trust but also positions the pharmaceutical isolator market as a critical component in the production process.
Rising Demand for Biopharmaceuticals
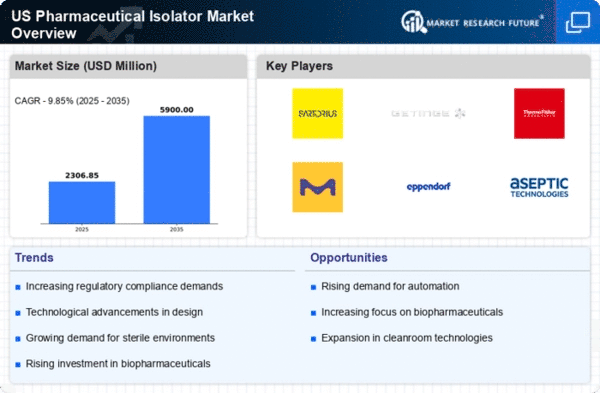
The increasing demand for biopharmaceuticals is a key driver for the pharmaceutical isolator market. As the biopharmaceutical sector expands, the need for aseptic environments becomes critical. This is particularly relevant in the production of monoclonal antibodies and vaccines, which require stringent contamination control. The pharmaceutical isolator market is projected to grow at a CAGR of approximately 9.85% from 2025 to 2030, driven by this demand. Companies are investing heavily in isolator technology to ensure compliance with industry standards, thereby enhancing production efficiency and product safety. The rise in chronic diseases and the need for innovative therapies further fuel this trend, indicating a robust future for the pharmaceutical isolator market as it adapts to meet the evolving needs of biopharmaceutical production.
Technological Innovations in Isolator Design
Technological innovations play a crucial role in shaping the pharmaceutical isolator market. Recent advancements in isolator design, such as the integration of automation and real-time monitoring systems, enhance operational efficiency and safety. These innovations allow for better control of environmental conditions, which is essential for maintaining sterility in pharmaceutical production. The market is witnessing a shift towards more sophisticated isolators that offer improved ergonomics and user-friendly interfaces. As a result, manufacturers are likely to invest in these advanced systems, which could lead to a market growth rate of around 12% over the next five years. The continuous evolution of technology in isolator design is expected to drive the pharmaceutical isolator market forward, catering to the increasing demands of the industry.
Growing Investment in Research and Development
Investment in research and development (R&D) is a significant driver for the pharmaceutical isolator market. As pharmaceutical companies seek to innovate and develop new therapies, the need for advanced isolator systems becomes more pronounced. R&D activities require controlled environments to ensure the integrity of experimental results, which in turn drives the demand for isolators. The pharmaceutical isolator market is likely to benefit from the projected increase in R&D spending, which is expected to reach $200 billion by 2026 in the US. This trend suggests that as companies allocate more resources to R&D, the demand for isolators will rise, further solidifying their role in the pharmaceutical production landscape. The interplay between R&D investment and isolator technology is likely to foster a dynamic growth environment for the market.
Expansion of Contract Manufacturing Organizations
The expansion of contract manufacturing organizations (CMOs) is a notable driver of the pharmaceutical isolator market. As pharmaceutical companies increasingly outsource production to CMOs, the demand for isolators that meet stringent regulatory requirements grows. CMOs are adopting advanced isolator technology to ensure compliance and maintain high-quality standards in their manufacturing processes. This trend is expected to contribute to a market growth rate of approximately 9% over the next few years. The rise of CMOs reflects a broader shift in the industry towards flexible manufacturing solutions, which further emphasizes the importance of isolators in maintaining product safety and efficacy. The pharmaceutical isolator market is expected to thrive as CMOs continue to expand their capabilities and invest in state-of-the-art isolator systems.