Rising Healthcare Expenditure
The increasing healthcare expenditure in the United States is a significant driver of the US Mitral Valve Stenosis Market. As healthcare spending continues to rise, there is a greater focus on improving patient outcomes and investing in advanced medical technologies. According to the Centers for Medicare & Medicaid Services (CMS), national health expenditure is projected to grow at an average rate of 5.4% per year, reaching nearly $6 trillion by 2027. This financial commitment allows for the development and adoption of innovative treatments for mitral valve stenosis, including surgical interventions and minimally invasive procedures. Furthermore, higher healthcare spending may lead to improved access to care for patients, thereby increasing the demand for mitral valve stenosis treatments and contributing to market growth.
Advancements in Medical Technology
Technological advancements play a pivotal role in shaping the US Mitral Valve Stenosis Market. Innovations in medical devices, such as transcatheter mitral valve repair and replacement technologies, have revolutionized treatment options for patients. These minimally invasive procedures offer reduced recovery times and lower risks compared to traditional surgical methods. The introduction of advanced imaging techniques, such as 3D echocardiography, enhances the accuracy of diagnosis and treatment planning. As healthcare providers increasingly adopt these technologies, the market is likely to witness significant growth. Moreover, the US Food and Drug Administration's (FDA) expedited approval processes for breakthrough devices may further accelerate the availability of innovative treatments, thereby expanding the market landscape for mitral valve stenosis interventions.
Government Initiatives and Funding
Government initiatives and funding are crucial drivers of the US Mitral Valve Stenosis Market. The US government has implemented various programs aimed at improving cardiovascular health, which includes funding for research and development in heart disease treatments. The National Institutes of Health (NIH) allocates substantial resources to cardiovascular research, which may lead to new therapies and technologies for mitral valve stenosis. Additionally, public health campaigns aimed at raising awareness about heart health can encourage early diagnosis and treatment, potentially increasing the patient population seeking care. These initiatives not only support innovation but also enhance access to care, thereby contributing to the overall growth of the market. The collaboration between government entities and private sectors may further bolster advancements in the US Mitral Valve Stenosis Market.
Growing Patient Awareness and Education
Growing patient awareness and education regarding heart health is a vital driver of the US Mitral Valve Stenosis Market. As patients become more informed about the symptoms and risks associated with mitral valve stenosis, they are more likely to seek medical attention and treatment. Educational initiatives by healthcare organizations and advocacy groups play a crucial role in disseminating information about heart conditions. This heightened awareness can lead to earlier diagnosis and intervention, which is essential for effective management of the disease. Additionally, as patients become more engaged in their healthcare decisions, they may actively seek out advanced treatment options, further stimulating demand within the market. The emphasis on patient education is likely to have a lasting impact on the US Mitral Valve Stenosis Market.
Increasing Prevalence of Mitral Valve Stenosis
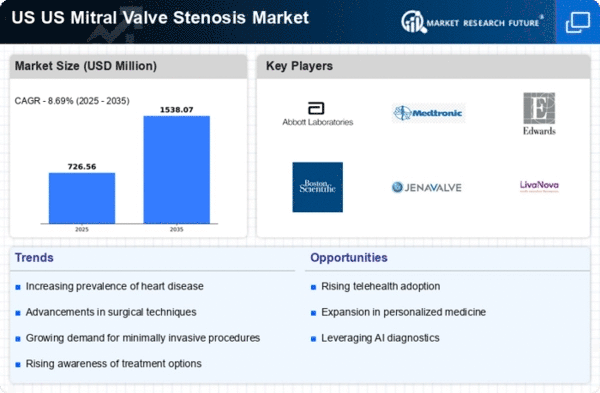
The US Mitral Valve Stenosis Market is experiencing growth due to the rising prevalence of mitral valve stenosis among the aging population. As individuals age, the risk of developing heart conditions, including mitral valve stenosis, increases significantly. According to the American Heart Association, approximately 2.5% of the population over 75 years old is affected by this condition. This demographic shift is likely to drive demand for diagnostic and therapeutic interventions, thereby expanding the market. Furthermore, the increasing awareness of heart health and the importance of early diagnosis may lead to more patients seeking treatment, which could further stimulate market growth. The healthcare system's focus on managing chronic conditions, including heart diseases, aligns with the growing need for effective solutions in the US Mitral Valve Stenosis Market.