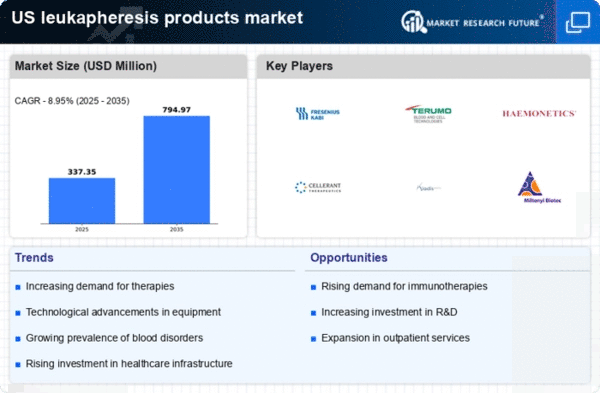
Rising Incidence of Chronic Diseases
The leukapheresis products market is being propelled by the rising incidence of chronic diseases, particularly those affecting the blood and immune systems. Conditions such as cancer, autoimmune disorders, and blood-related diseases are becoming increasingly prevalent, necessitating the use of leukapheresis for therapeutic interventions. In 2025, it is estimated that over 1.5 million new cancer cases will be diagnosed in the US, highlighting the urgent need for effective treatment options. Leukapheresis is often employed in conjunction with other therapies to manage these conditions, thereby driving demand for related products. As healthcare providers seek to offer comprehensive treatment solutions, the leukapheresis products market is likely to expand in response to the growing burden of chronic diseases.
Increasing Demand for Blood Components
The leukapheresis products market is experiencing a notable surge in demand for blood components, driven by the rising prevalence of hematological disorders and the need for blood transfusions. As the population ages, the incidence of conditions such as leukemia and lymphoma is increasing, necessitating the use of leukapheresis to collect specific blood components. In 2025, the market for blood components is projected to reach approximately $5 billion, with leukapheresis playing a crucial role in meeting this demand. This trend indicates a growing reliance on leukapheresis products to ensure a steady supply of vital blood components, thereby propelling the market forward. Furthermore, advancements in collection techniques and equipment are likely to enhance the efficiency of leukapheresis procedures, further stimulating market growth.
Growing Investment in Research and Development
Investment in research and development (R&D) is a critical driver for the leukapheresis products market, as companies strive to innovate and improve existing products. Increased funding from both public and private sectors is facilitating the development of novel leukapheresis technologies and methodologies. In 2025, R&D spending in the healthcare sector is projected to exceed $200 billion, with a significant portion allocated to blood management technologies. This influx of investment is likely to lead to breakthroughs in leukapheresis procedures, enhancing their effectiveness and safety. Furthermore, collaborations between academic institutions and industry players are expected to yield new insights and advancements, thereby fostering growth in the leukapheresis products market. The focus on R&D underscores the commitment to improving patient care and outcomes in hematological treatments.
Technological Innovations in Apheresis Equipment
Technological innovations are significantly shaping the leukapheresis products market, as advancements in apheresis equipment enhance the efficiency and safety of procedures. The introduction of automated systems and improved filtration technologies has streamlined the leukapheresis process, reducing the time required for blood component collection. In 2025, the market for apheresis devices is expected to grow at a CAGR of 8%, reflecting the increasing adoption of these advanced technologies. Moreover, the integration of real-time monitoring systems ensures better patient outcomes and minimizes complications, which is likely to attract more healthcare facilities to adopt leukapheresis products. As hospitals and clinics seek to improve their operational efficiency, the demand for innovative apheresis equipment is anticipated to rise, thereby driving the overall market.
Enhanced Regulatory Support for Apheresis Procedures
Enhanced regulatory support for apheresis procedures is emerging as a key driver for the leukapheresis products market. Regulatory bodies are increasingly recognizing the importance of leukapheresis in therapeutic applications, leading to streamlined approval processes for new products and technologies. In 2025, it is anticipated that regulatory frameworks will continue to evolve, providing clearer guidelines for the development and use of leukapheresis products. This supportive environment is likely to encourage innovation and investment in the market, as companies seek to bring new solutions to the forefront. Additionally, the establishment of best practice guidelines by regulatory agencies is expected to improve the safety and efficacy of leukapheresis procedures, further bolstering market growth.