Advancements in Biotechnology
Technological advancements in biotechnology are significantly influencing the interleukin market. Innovations in genetic engineering, monoclonal antibody production, and recombinant DNA technology have enabled the development of novel interleukin therapies. These advancements facilitate the creation of more targeted and effective treatments, which are essential for managing complex diseases. The US biotechnology sector has seen substantial growth, with investments exceeding $50 billion in recent years. This influx of capital is likely to accelerate research and development efforts in the interleukin market, leading to the introduction of new products. Furthermore, the integration of artificial intelligence in drug discovery processes may enhance the efficiency of identifying promising interleukin candidates, thereby driving market expansion. As a result, the interleukin market is poised for significant advancements in therapeutic options.
Growing Awareness of Immunotherapy
The increasing awareness and acceptance of immunotherapy as a treatment modality is a notable driver for the interleukin market. Healthcare professionals and patients are becoming more informed about the benefits of harnessing the immune system to combat diseases, particularly cancer. Interleukin therapies, which enhance immune responses, are gaining traction in oncology, leading to a surge in clinical trials and approvals. The US market for immunotherapy is projected to grow at a CAGR of approximately 15% over the next five years, indicating a robust demand for interleukin-based treatments. This trend is likely to encourage pharmaceutical companies to invest in the development of new interleukin therapies, further propelling the interleukin market. As more patients seek these innovative treatments, the market is expected to expand significantly.
Rising Demand for Targeted Therapies
The shift towards personalized and targeted therapies is a prominent driver for the interleukin market. Patients and healthcare providers are increasingly seeking treatments that are tailored to individual genetic profiles and specific disease mechanisms. Interleukin therapies, which can be designed to target particular pathways in the immune system, align well with this trend. The US market for targeted therapies is expected to grow substantially, with projections indicating a CAGR of around 12% through 2030. This growing demand is likely to prompt pharmaceutical companies to invest in research and development of interleukin-based treatments that offer improved efficacy and reduced side effects. Consequently, the interleukin market is anticipated to benefit from this trend, as more innovative and effective therapies become available.
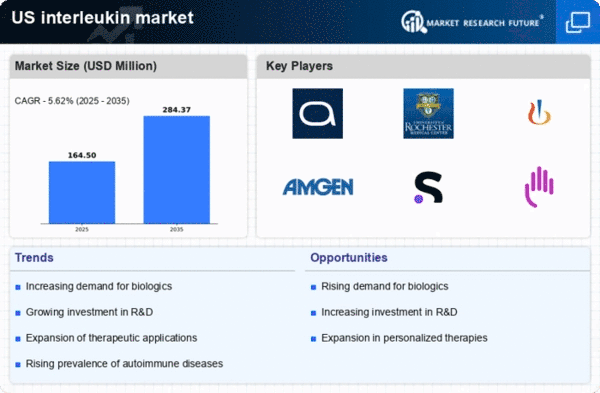
Increasing Prevalence of Autoimmune Diseases
The rising incidence of autoimmune diseases in the US is a critical driver for the interleukin market. Conditions such as rheumatoid arthritis, lupus, and multiple sclerosis are becoming more common, leading to a heightened demand for effective treatments. According to recent estimates, autoimmune diseases affect approximately 5-8% of the US population, which translates to millions of individuals seeking therapeutic options. This growing patient population is likely to stimulate investment in interleukin-based therapies, as these cytokines play a pivotal role in immune regulation. The interleukin market is expected to expand as pharmaceutical companies focus on developing innovative therapies targeting specific interleukins associated with these diseases. As a result, the market could witness substantial growth, potentially reaching a valuation of over $10 billion by 2027.
Regulatory Incentives for Biopharmaceuticals
Regulatory incentives provided by the US government for biopharmaceuticals are fostering growth in the interleukin market. Initiatives such as the Orphan Drug Act and the 21st Century Cures Act encourage the development of therapies for rare diseases and streamline the approval process for new drugs. These incentives are particularly beneficial for interleukin therapies, which often target niche markets with unmet medical needs. The potential for expedited approval and market exclusivity can significantly enhance the profitability of interleukin products. As a result, pharmaceutical companies are likely to increase their focus on developing interleukin-based therapies, contributing to the overall growth of the interleukin market. This supportive regulatory environment may lead to a more dynamic and competitive landscape in the coming years.