Rising Awareness and Education
Increased awareness and education regarding benign prostatic hyperplasia are driving growth in the benign prostatic-hyperplasia-treatment market. Campaigns aimed at educating men about BPH symptoms and treatment options have led to a greater understanding of the condition. This heightened awareness encourages men to seek medical advice earlier, resulting in timely interventions. Healthcare providers are increasingly focusing on patient education, which is essential for improving treatment adherence and outcomes. As more men become informed about the potential complications of untreated BPH, the demand for effective treatment options is likely to rise. This trend is expected to positively impact the benign prostatic-hyperplasia-treatment market, as more patients actively pursue solutions to manage their symptoms.
Increasing Healthcare Expenditure
The benign prostatic-hyperplasia-treatment market is also influenced by rising healthcare expenditure in the United States. As healthcare spending continues to grow, patients are more likely to seek medical attention for conditions like BPH. The U.S. healthcare expenditure is projected to reach approximately $6 trillion by 2027, which may facilitate access to advanced treatment options for BPH. Increased funding for research and development in urology is likely to lead to the introduction of novel therapies and improved treatment protocols. This financial commitment to healthcare is expected to enhance the benign prostatic-hyperplasia-treatment market, as more resources become available for patient care and innovative treatment solutions.
Advancements in Medical Technology
Technological advancements play a crucial role in shaping the benign prostatic-hyperplasia-treatment market. Innovations in minimally invasive surgical techniques, such as laser therapy and transurethral resection of the prostate (TURP), have improved patient outcomes and reduced recovery times. These advancements not only enhance the efficacy of treatments but also attract more patients seeking effective solutions for BPH. The market is witnessing a growing adoption of robotic-assisted surgeries, which offer precision and reduced complications. Furthermore, the integration of digital health technologies, including telehealth platforms, is facilitating better patient management and follow-up care. As these technologies continue to evolve, they are likely to contribute to the expansion of the benign prostatic-hyperplasia-treatment market, making treatments more accessible and efficient.
Aging Population and Increased Incidence
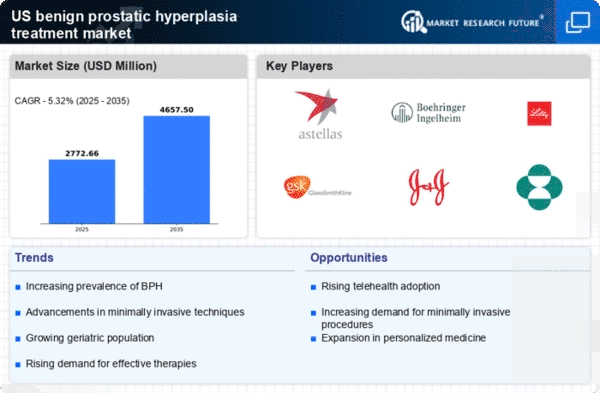
The benign prostatic-hyperplasia-treatment market is significantly influenced by the aging population in the United States. As men age, the likelihood of developing benign prostatic hyperplasia (BPH) increases, with studies indicating that approximately 50% of men aged 50 and older experience some degree of BPH. This demographic shift is expected to drive demand for treatment options, as the population aged 65 and older is projected to reach 80 million by 2040. Consequently, healthcare providers are likely to see a surge in patients seeking interventions for BPH, thereby propelling growth in the benign prostatic-hyperplasia-treatment market. The increasing prevalence of BPH among older men necessitates a variety of treatment modalities, including medications and surgical options, further expanding the market landscape.
Regulatory Support and Approval of New Treatments
Regulatory support for the approval of new treatments is a vital driver of the benign prostatic-hyperplasia-treatment market. The U.S. Food and Drug Administration (FDA) plays a crucial role in evaluating and approving new therapies for BPH. Recent approvals of novel medications and devices have expanded the treatment landscape, providing patients with more options. The FDA's streamlined processes for innovative therapies may encourage pharmaceutical companies to invest in research and development for BPH treatments. As new therapies enter the market, competition is likely to increase, potentially leading to more effective and affordable treatment options for patients. This regulatory environment is expected to foster growth in the benign prostatic-hyperplasia-treatment market.