Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies in the arteriovenous malformation market is emerging as a key driver. The US Food and Drug Administration (FDA) has been increasingly supportive of novel treatment approaches, expediting the approval process for breakthrough therapies. This regulatory environment encourages pharmaceutical and medical device companies to invest in research and development for AVM treatments. As a result, the arteriovenous malformation market is likely to see a proliferation of new products and therapies entering the market, which could enhance treatment options for patients and stimulate overall market growth.
Increased Awareness and Education Initiatives
Increased awareness and education initiatives regarding arteriovenous malformations are contributing to market growth. Various organizations and healthcare providers are actively promoting knowledge about AVMs, their symptoms, and treatment options. This heightened awareness is likely to lead to earlier diagnosis and intervention, which could improve patient outcomes. The arteriovenous malformation market benefits from these initiatives as they encourage more individuals to seek medical advice, thereby increasing the demand for diagnostic and therapeutic services. As educational campaigns continue to expand, the market may experience a corresponding rise in patient engagement and treatment uptake.
Growing Investment in Healthcare Infrastructure
The growing investment in healthcare infrastructure in the US is a crucial driver for the arteriovenous malformation market. Increased funding for hospitals and specialized clinics facilitates the acquisition of advanced medical equipment and technologies necessary for the diagnosis and treatment of AVMs. According to recent data, healthcare spending in the US is projected to reach approximately $4 trillion by 2025, which may enhance the capabilities of healthcare providers in managing complex conditions like AVMs. This investment is likely to bolster the arteriovenous malformation market by improving access to care and expanding treatment options for patients.
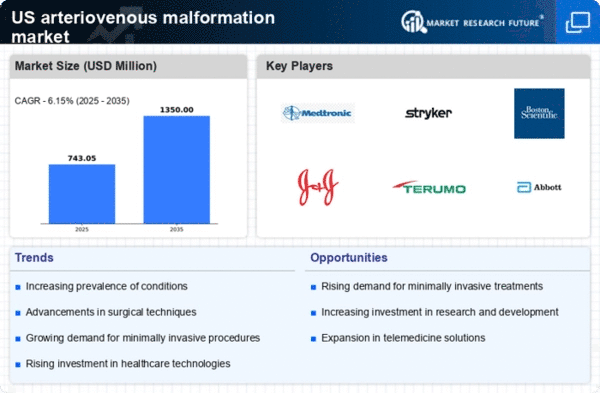
Rising Incidence of Arteriovenous Malformations
The increasing incidence of arteriovenous malformations (AVMs) in the population appears to be a significant driver for the arteriovenous malformation market. Recent estimates suggest that AVMs affect approximately 1-2 individuals per 100,000 people annually in the US. This rising prevalence necessitates enhanced diagnostic and therapeutic options, thereby propelling market growth. As awareness of AVMs increases, more patients are likely to seek medical attention, leading to a higher demand for treatment modalities. The arteriovenous malformation market is thus positioned to expand as healthcare providers focus on developing innovative solutions to address this growing health concern.
Technological Advancements in Treatment Options
Technological advancements in treatment options for arteriovenous malformations are likely to drive the market forward. Innovations such as endovascular embolization and stereotactic radiosurgery have shown promising results in managing AVMs. The arteriovenous malformation market is witnessing a surge in research and development activities aimed at improving these techniques. For instance, the introduction of new embolic agents and imaging technologies has enhanced the precision of AVM treatments. As these technologies become more widely adopted, they could potentially lead to better patient outcomes and increased market penetration, thereby fostering growth in the industry.