Advancements in Telemedicine
The aicardi syndrome market is being positively influenced by advancements in telemedicine, which have transformed how healthcare is delivered. In the UK, telehealth services have expanded access to specialists for patients with rare conditions, including aicardi syndrome. This shift allows for timely consultations and follow-ups, reducing the burden on families who may have to travel long distances for care. As telemedicine continues to evolve, it is expected to enhance patient engagement and adherence to treatment plans. This trend may lead to improved health outcomes and increased demand for services within the aicardi syndrome market, as more families seek accessible and efficient care options.
Increased Research Collaboration
The aicardi syndrome market is benefiting from increased collaboration among researchers, healthcare providers, and patient advocacy groups. Collaborative efforts are essential for advancing understanding of the disorder and developing effective treatments. In the UK, partnerships between academic institutions and pharmaceutical companies are fostering innovation in drug development and clinical trials. This collaborative environment is likely to accelerate the discovery of new therapies and improve patient care. Additionally, shared resources and knowledge can enhance the overall landscape of the aicardi syndrome market, leading to better outcomes for patients and families affected by this condition.
Government Initiatives and Funding
The UK government has been proactive in addressing rare diseases, including aicardi syndrome, through various initiatives and funding programs. The establishment of the Rare Diseases Framework aims to improve diagnosis, treatment, and care for patients with rare conditions. Increased funding for research and healthcare services is likely to enhance the aicardi syndrome market, as it facilitates the development of new therapies and support systems. Furthermore, public health campaigns aimed at raising awareness about rare diseases may lead to earlier diagnosis and intervention, ultimately benefiting patients and their families. This supportive environment is crucial for the growth of the aicardi syndrome market.
Rising Incidence of Aicardi Syndrome
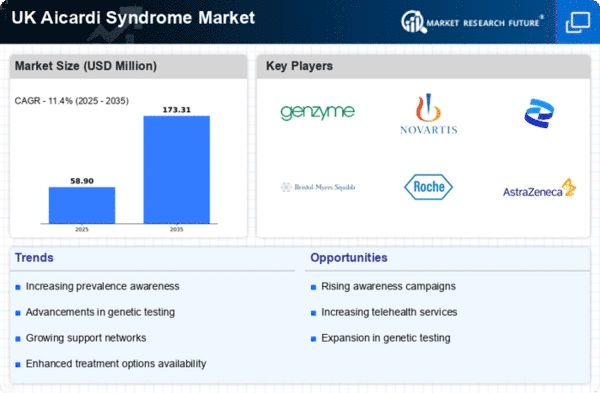
The aicardi syndrome market is experiencing growth due to an observed increase in the incidence of this rare genetic disorder. Recent studies indicate that the prevalence of aicardi syndrome in the UK is approximately 1 in 100,000 live births. This rising incidence necessitates enhanced healthcare services and resources dedicated to diagnosis and treatment. As awareness grows among healthcare professionals and the general public, more cases are likely to be identified, leading to a greater demand for specialized care. Consequently, this trend is expected to drive investments in research and development within the aicardi syndrome market, fostering innovation in treatment options and improving patient outcomes.
Growing Patient Advocacy and Support Networks
The aicardi syndrome market is witnessing a rise in patient advocacy and support networks, which play a crucial role in raising awareness and providing resources for affected families. In the UK, organizations dedicated to rare diseases are increasingly active in promoting research, funding initiatives, and offering support services. These networks not only empower patients and families but also facilitate communication between stakeholders in the aicardi syndrome market. As advocacy efforts grow, they are likely to influence policy changes and funding allocations, ultimately enhancing the quality of care and support available to those affected by aicardi syndrome.