Expansion of Testing Laboratories
The Specimen Validity Testing Market is benefiting from the expansion of testing laboratories that specialize in drug testing services. As the demand for accurate and reliable testing increases, more laboratories are emerging to provide comprehensive specimen validity testing solutions. This expansion is driven by the need for timely and precise results in various sectors, including healthcare, law enforcement, and corporate environments. Furthermore, advancements in laboratory technology and the establishment of accredited testing facilities are enhancing the overall quality of testing services. Market analysis suggests that the number of accredited laboratories is expected to rise by approximately 15% in the coming years, indicating a robust growth trajectory for the specimen validity testing market.
Regulatory Compliance and Standards
The Specimen Validity Testing Market is significantly influenced by stringent regulatory compliance and standards imposed by various health and safety organizations. These regulations mandate the implementation of specimen validity testing to ensure the integrity of drug testing processes. For example, the Substance Abuse and Mental Health Services Administration (SAMHSA) has established guidelines that require testing laboratories to perform validity tests on specimens. This regulatory framework not only enhances the credibility of testing results but also fosters a culture of accountability among employers and testing facilities. Consequently, the market is expected to expand as organizations increasingly prioritize compliance with these regulations, thereby driving demand for advanced testing solutions.
Rising Awareness of Substance Abuse
The Specimen Validity Testing Market is witnessing growth fueled by the rising awareness of substance abuse and its implications on public health and safety. As communities become more cognizant of the effects of drug misuse, there is a growing demand for effective testing solutions to identify substance abuse in various settings, including workplaces and educational institutions. This heightened awareness has led to an increase in the implementation of drug testing programs, which often incorporate specimen validity testing to ensure the authenticity of results. Market data indicates that the prevalence of drug testing in workplaces has increased by over 20% in recent years, reflecting a proactive approach to managing substance abuse issues. This trend is likely to continue, further propelling the growth of the specimen validity testing market.
Increasing Employment Drug Testing Policies
The Specimen Validity Testing Market is also driven by the increasing adoption of employment drug testing policies across various sectors. Employers are increasingly recognizing the importance of maintaining a drug-free workplace to enhance productivity and ensure safety. As a result, many organizations are implementing comprehensive drug testing programs that include specimen validity testing to prevent tampering and ensure accurate results. According to recent statistics, nearly 70% of employers in certain industries have adopted drug testing policies, which has led to a surge in demand for reliable testing solutions. This trend is expected to persist, as more companies seek to mitigate risks associated with substance abuse, thereby contributing to the growth of the specimen validity testing market.
Technological Advancements in Testing Methods
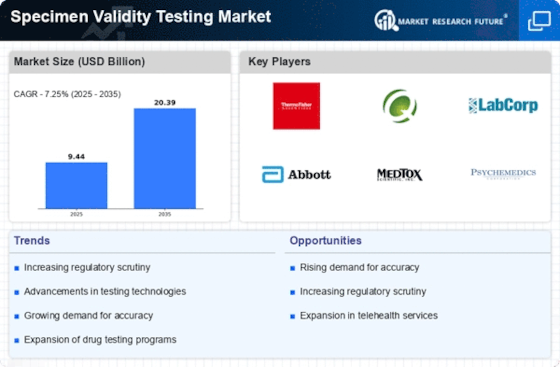
The Specimen Validity Testing Market is experiencing a notable transformation due to rapid technological advancements. Innovations in testing methods, such as the introduction of automated systems and advanced analytical techniques, enhance the accuracy and efficiency of specimen analysis. For instance, the integration of mass spectrometry and immunoassays has improved the detection of adulterants and substitutions in urine samples. This technological evolution not only streamlines the testing process but also reduces the turnaround time for results, which is crucial for employers and regulatory bodies. As a result, the market is projected to grow at a compound annual growth rate (CAGR) of approximately 7% over the next five years, driven by the demand for reliable and efficient testing solutions.
.png)