Growing Healthcare Expenditure
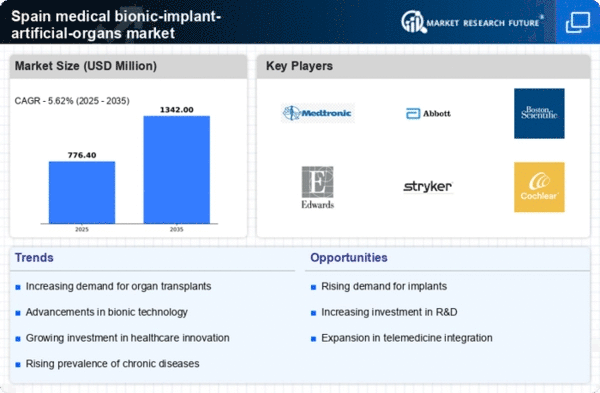
The upward trend in healthcare expenditure in Spain significantly influences the medical bionic-implant-artificial-organs market. In 2025, healthcare spending is projected to account for approximately 10% of the national GDP, reflecting a commitment to improving health services and patient care. This increase in funding allows for the integration of advanced medical technologies, including bionic implants and artificial organs, into healthcare systems. Hospitals and clinics are more likely to invest in these innovative solutions, enhancing their service offerings. Additionally, the rising demand for personalized medicine and tailored treatments further propels the market, as healthcare providers seek to adopt technologies that align with patient needs. Consequently, the medical bionic-implant-artificial-organs market stands to gain from this favorable economic environment.
Rising Incidence of Chronic Diseases
The increasing prevalence of chronic diseases in Spain is a pivotal driver for the medical bionic-implant-artificial-organs market. Conditions such as diabetes, heart disease, and renal failure necessitate advanced medical interventions. According to recent health statistics, approximately 25% of the Spanish population suffers from chronic ailments, leading to a heightened demand for innovative solutions. The medical bionic-implant-artificial-organs market is poised to benefit from this trend, as patients seek alternatives to traditional treatments. Furthermore, the aging population, which is projected to reach 30% by 2030, exacerbates the need for effective medical interventions. This demographic shift indicates a growing market potential, as older individuals are more susceptible to chronic conditions requiring bionic implants and artificial organs.
Investment in Research and Development
Investment in research and development (R&D) is crucial for the advancement of the medical bionic-implant-artificial-organs market. In Spain, government and private sector funding for biomedical research has seen a notable increase, with allocations reaching €1 billion in 2025. This financial support fosters innovation, enabling the development of cutting-edge technologies and materials for bionic implants. Collaborations between universities, research institutions, and healthcare companies are becoming more prevalent, enhancing the knowledge base and accelerating product development. As a result, The market is likely to witness a surge in novel products that improve patient outcomes and expand treatment options. The emphasis on R&D not only drives technological advancements but also positions Spain as a competitive player in the global market.
Technological Integration in Healthcare
The integration of technology in healthcare practices is transforming the medical bionic-implant-artificial-organs market. In Spain, the adoption of digital health solutions, such as telemedicine and electronic health records, is on the rise, facilitating better patient management and data sharing. This technological shift enhances the efficiency of healthcare delivery, allowing for timely interventions and improved patient outcomes. Moreover, advancements in materials science and engineering are leading to the development of more biocompatible and durable bionic implants. As healthcare providers increasingly embrace these technologies, the medical bionic-implant-artificial-organs market is likely to expand, driven by the demand for innovative solutions that enhance the quality of care. The synergy between technology and healthcare is expected to yield significant benefits for patients and providers alike.
Increased Collaboration Among Stakeholders
The medical bionic-implant-artificial-organs market is experiencing a surge in collaboration among various stakeholders, including healthcare providers, manufacturers, and regulatory bodies. In Spain, partnerships between public and private sectors are fostering innovation and streamlining the development process for bionic implants and artificial organs. These collaborations often result in shared resources, knowledge exchange, and accelerated product development timelines. Furthermore, regulatory bodies are increasingly engaging with industry players to establish clear guidelines and standards, which enhances market confidence. This collaborative environment not only drives innovation but also ensures that new products meet safety and efficacy requirements. As a result, the medical bionic-implant-artificial-organs market is likely to benefit from a more cohesive approach to development and implementation.