South Korea Safety Lancet Market Summary
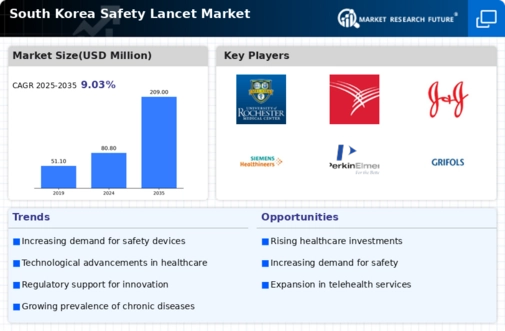
The South Korea Safety Lancet Market is poised for substantial growth, reaching 209 million USD by 2035 from 80.8 million USD in 2024.
Key Market Trends & Highlights
South Korea Safety Lancet Market Key Trends and Highlights
- The market valuation is projected to grow from 80.8 million USD in 2024 to 209 million USD by 2035.
- A compound annual growth rate of 9.03 percent is expected from 2025 to 2035.
- The increasing focus on patient safety and quality of care is driving market expansion.
- Growing adoption of safety technologies due to heightened regulatory standards is a major market driver.
Market Size & Forecast
| 2024 Market Size | 80.8 (USD Million) |
| 2035 Market Size | 209 (USD Million) |
| CAGR (2025-2035) | 9.03% |
Major Players
Medtronic, Roche, Hirschmann Automation and Control, Nipro Corporation, Quidel Corporation, Cardinal Health, Johnson & Johnson, F. Hoffmann La Roche, Becton Dickinson, Siemens Healthineers, PerkinElmer, Grifols, Abbott, Sekisui Medical, Thermo Fisher Scientific