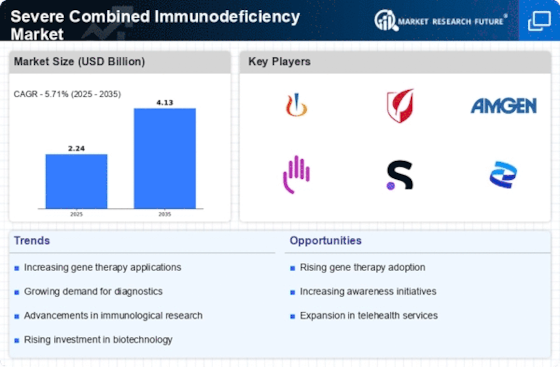
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a significant factor influencing the Severe Combined Immunodeficiency Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for treatments targeting rare diseases like SCID. Initiatives such as orphan drug designations and fast-track approvals are designed to encourage the development of new therapies. This regulatory environment is fostering innovation and attracting investment in the SCID treatment landscape. As a result, pharmaceutical companies are more likely to invest in research and development, leading to a broader array of treatment options for patients. The supportive regulatory framework is expected to enhance market growth by facilitating quicker access to life-saving therapies.
Growing Patient Advocacy and Support Networks
The emergence of patient advocacy and support networks is playing a pivotal role in shaping the Severe Combined Immunodeficiency Market. These organizations are instrumental in raising awareness about SCID, promoting early diagnosis, and advocating for better treatment options. Their efforts have led to increased visibility of the disease, which may result in higher funding for research and development. Furthermore, these networks often collaborate with healthcare providers and policymakers to influence legislation that supports SCID patients. As the patient community becomes more organized and vocal, it is likely to drive demand for innovative therapies and improve access to care, thereby positively impacting the market.
Increased Investment in Rare Disease Research
Increased investment in rare disease research is a critical driver for the Severe Combined Immunodeficiency Market. Governments and private organizations are recognizing the need for targeted research funding to address the challenges posed by rare diseases like SCID. This trend is reflected in the allocation of resources towards developing novel therapies and improving diagnostic tools. For example, funding initiatives have surged, with billions of dollars being directed towards research in gene therapy and immunology. Such investments are expected to yield new treatment options, thereby enhancing the market landscape. The focus on rare diseases is likely to foster collaboration among stakeholders, including pharmaceutical companies and research institutions, which may further accelerate advancements in SCID treatment.
Technological Advancements in Treatment Modalities
Technological advancements in treatment modalities are significantly influencing the Severe Combined Immunodeficiency Market. Innovations such as gene therapy and stem cell transplantation are revolutionizing the way SCID is treated. For instance, recent developments in gene editing techniques, like CRISPR, have shown promise in correcting genetic defects associated with SCID. The market for gene therapy is projected to grow substantially, with estimates indicating a compound annual growth rate of over 30% in the coming years. These advancements not only enhance treatment efficacy but also improve patient outcomes, thereby driving market growth. As new technologies emerge, they are likely to attract investment and research, further propelling the market forward.
Rising Incidence of Severe Combined Immunodeficiency
The increasing incidence of Severe Combined Immunodeficiency Market (SCID) is a notable driver in the Severe Combined Immunodeficiency Market. Recent estimates suggest that SCID affects approximately 1 in 58,000 live births, indicating a growing population of patients requiring treatment. This rise in incidence is likely to spur demand for innovative therapies and interventions, thereby expanding the market. As awareness of SCID improves, more cases are being diagnosed, which may lead to an increase in treatment options and healthcare investments. The need for effective management strategies for SCID patients is becoming more pressing, suggesting that the market will continue to evolve in response to these demographic changes.