Rising Healthcare Expenditure
The rise in global healthcare expenditure is a notable driver for the Global RSV Diagnostics Market Industry. As countries invest more in healthcare infrastructure and services, there is an increased focus on improving diagnostic capabilities for infectious diseases, including RSV. This trend is particularly evident in developing nations, where healthcare systems are evolving to meet the growing demand for advanced diagnostic solutions. Increased funding for healthcare initiatives allows for the procurement of state-of-the-art diagnostic equipment and technologies. As a result, the market is expected to benefit from this upward trend in healthcare spending, facilitating the development and distribution of effective RSV diagnostic tools.
Government Initiatives and Funding
Government initiatives and funding play a crucial role in shaping the Global RSV Diagnostics Market Industry. Various health organizations and governmental bodies are increasingly recognizing the need for improved RSV diagnostics, leading to the allocation of resources for research and development. For example, public health campaigns aimed at raising awareness about RSV and its impact on vulnerable populations have been initiated in several countries. Additionally, funding for innovative diagnostic solutions is being provided to encourage the development of more efficient testing methods. Such initiatives are expected to foster market growth, as they create a conducive environment for the introduction of novel diagnostic technologies.
Increasing Incidence of RSV Infections
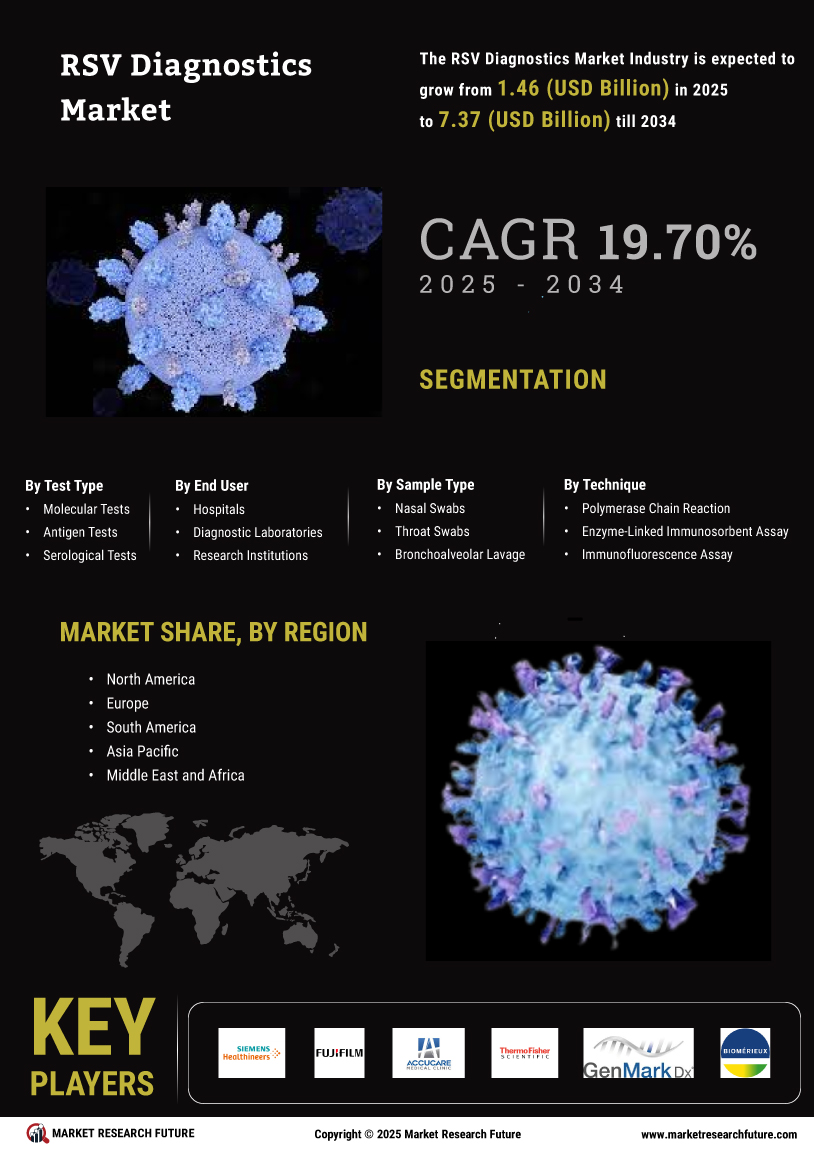
The rising incidence of respiratory syncytial virus infections is a primary driver for the Global RSV Diagnostics Market Industry. In recent years, the prevalence of RSV has been notably high among infants and elderly populations, leading to increased hospitalizations and healthcare costs. For instance, it is estimated that RSV causes approximately 1.5 million hospitalizations annually in children under five years old globally. This growing burden of disease necessitates the development and adoption of effective diagnostic tools, thereby propelling market growth. As the industry evolves, the demand for rapid and accurate RSV diagnostics is expected to surge, contributing to the projected market value of 1.22 USD Billion in 2024.
Technological Advancements in Diagnostic Tools
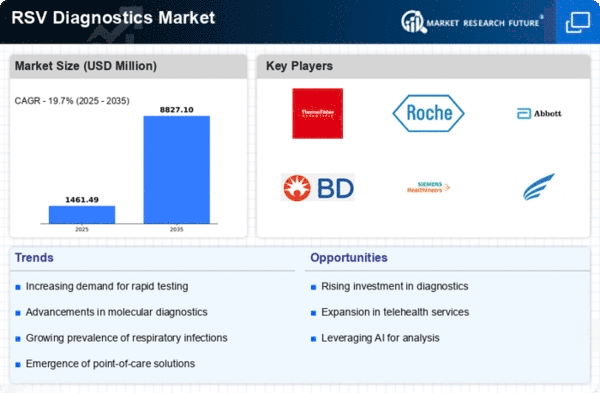
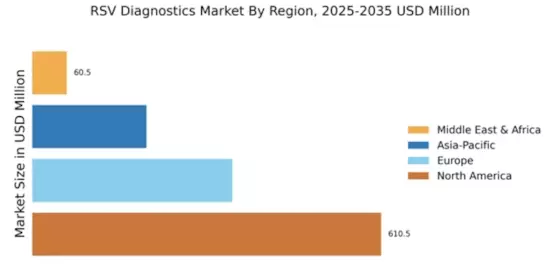
Technological innovations in diagnostic tools significantly influence the Global RSV Diagnostics Market Industry. The introduction of molecular diagnostic techniques, such as PCR and next-generation sequencing, enhances the accuracy and speed of RSV detection. These advancements not only facilitate timely diagnosis but also improve patient management strategies. Furthermore, the integration of point-of-care testing devices allows for rapid testing in various healthcare settings, including outpatient clinics and emergency departments. As these technologies become more widely adopted, they are likely to contribute to the market's growth trajectory, with expectations of reaching 8.82 USD Billion by 2035, reflecting a compound annual growth rate of 19.7% from 2025 to 2035.
Growing Awareness of RSV Among Healthcare Professionals
The growing awareness of RSV among healthcare professionals is another significant driver for the Global RSV Diagnostics Market Industry. As healthcare providers become more informed about the clinical implications of RSV infections, there is an increasing emphasis on early diagnosis and treatment. This heightened awareness leads to a greater demand for reliable diagnostic tools that can accurately identify RSV in patients. Educational programs and training sessions aimed at healthcare professionals are being implemented to enhance their understanding of RSV and its management. Consequently, this trend is likely to stimulate market growth as the demand for effective RSV diagnostics continues to rise.