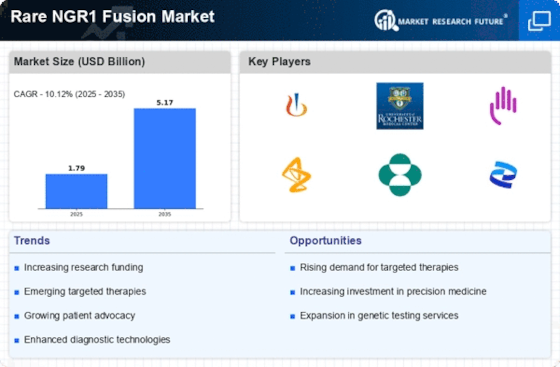
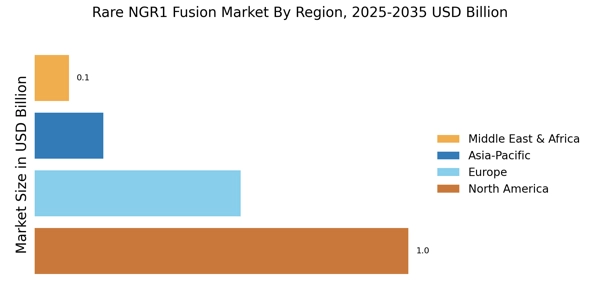
Advancements in Genomic Research
The rapid advancements in genomic research are significantly influencing the Rare NGR1 Fusion Market. Innovations in sequencing technologies, such as next-generation sequencing (NGS), have made it feasible to identify rare genetic fusions with greater accuracy and speed. This has led to a surge in research initiatives aimed at understanding the underlying mechanisms of NGR1 fusions and their implications for treatment. According to recent studies, the market for genomic testing is projected to reach USD 25 billion by 2026, reflecting the growing investment in this area. As researchers uncover more about the genetic landscape of rare diseases, the demand for targeted therapies tailored to NGR1 fusion patients is expected to increase, thereby propelling market growth.
Increased Awareness and Advocacy
The heightened awareness and advocacy surrounding rare diseases are driving growth in the Rare NGR1 Fusion Market. Patient advocacy groups and non-profit organizations are playing a vital role in raising awareness about rare genetic conditions, including NGR1 fusions. Their efforts are not only educating the public but also influencing policy changes and funding allocations for research. Recent surveys indicate that nearly 70% of the public is now aware of rare diseases, which is a significant increase compared to previous years. This growing awareness is likely to lead to increased demand for diagnostic testing and targeted therapies, as patients and healthcare providers seek effective treatment options. Consequently, the market for NGR1 fusion therapies may experience substantial growth as awareness continues to expand.
Growing Investment in Precision Medicine
The increasing investment in precision medicine is a crucial driver for the Rare NGR1 Fusion Market. As healthcare stakeholders recognize the potential of personalized therapies, funding for research and development in this field has surged. Reports indicate that the precision medicine market is anticipated to exceed USD 100 billion by 2025, highlighting the financial commitment to developing tailored treatments for rare genetic conditions, including those involving NGR1 fusions. This influx of capital is likely to foster innovation, leading to the emergence of novel therapeutic options that specifically target the unique characteristics of NGR1 fusion cases. Consequently, this trend may enhance treatment efficacy and improve patient outcomes, further stimulating market growth.
Regulatory Support for Rare Disease Treatments
Regulatory support for treatments targeting rare diseases is becoming increasingly robust, which is beneficial for the Rare NGR1 Fusion Market. Governments and regulatory bodies are implementing policies that expedite the approval process for orphan drugs, thereby encouraging pharmaceutical companies to invest in research for rare genetic conditions. For instance, the Orphan Drug Act in various regions provides incentives such as tax credits and market exclusivity for companies developing therapies for rare diseases. This supportive regulatory environment is likely to enhance the development pipeline for NGR1 fusion treatments, as companies are more inclined to pursue these opportunities. As a result, the market may witness a rise in the availability of effective therapies, ultimately benefiting patients with rare NGR1 fusions.
Increasing Prevalence of Rare NGR1 Fusion Cases
The rising incidence of rare NGR1 fusion cases is a pivotal driver for the Rare NGR1 Fusion Market. As healthcare systems enhance their diagnostic capabilities, more cases are being identified, leading to a greater demand for targeted therapies. Recent data indicates that the prevalence of rare genetic fusions, including NGR1, has been on the rise, with estimates suggesting that approximately 1 in 10,000 individuals may be affected. This growing recognition of rare NGR1 fusion cases is likely to stimulate research and development efforts, thereby expanding the market for innovative treatment options. Furthermore, as awareness increases among healthcare professionals, the likelihood of early diagnosis improves, which could potentially lead to better patient outcomes and increased market growth.