Advancements in Biotechnology
Technological advancements in biotechnology are transforming the Rare Disease Treatment Market. Innovations such as gene therapy, CRISPR technology, and monoclonal antibodies are enabling the development of novel therapies tailored to specific genetic disorders. The market for gene therapies alone is projected to reach USD 13 billion by 2026, reflecting the potential of these cutting-edge treatments. As biotechnology continues to evolve, it is expected that more effective and personalized treatment options will emerge, further driving market expansion and improving patient outcomes.
Regulatory Support and Incentives
Regulatory frameworks and incentives play a crucial role in shaping the Rare Disease Treatment Market. Governments and regulatory bodies are increasingly implementing policies to encourage the development of orphan drugs, which are designed to treat rare diseases. For instance, the Orphan Drug Act in the United States provides tax credits, grants, and market exclusivity for companies developing treatments for rare conditions. Such supportive measures are likely to stimulate investment in research and development, fostering innovation and accelerating the availability of new therapies for patients.
Rising Prevalence of Rare Diseases
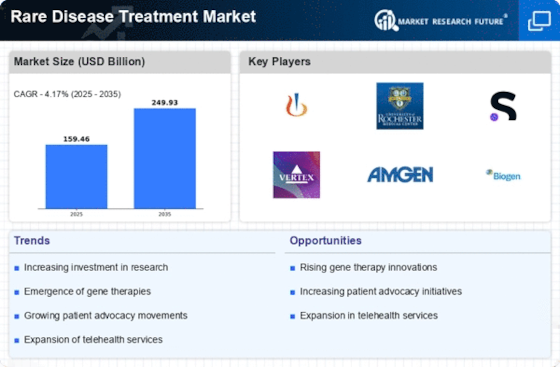
The increasing prevalence of rare diseases is a pivotal driver for the Rare Disease Treatment Market. It is estimated that approximately 7,000 distinct rare diseases exist, affecting around 400 million individuals worldwide. This growing patient population necessitates the development of targeted therapies and innovative treatment options. As awareness of these conditions rises, pharmaceutical companies are increasingly investing in research and development to address unmet medical needs. The demand for effective treatments is likely to propel market growth, as healthcare providers seek to offer solutions for patients suffering from these often debilitating conditions.
Increased Patient Advocacy and Awareness
The rise of patient advocacy groups and increased awareness surrounding rare diseases are driving forces in the Rare Disease Treatment Market. These organizations play a vital role in educating the public and healthcare professionals about rare conditions, fostering a sense of community among patients. As awareness grows, so does the demand for effective treatments, prompting pharmaceutical companies to prioritize research and development efforts. This heightened advocacy not only influences policy changes but also encourages collaboration between stakeholders, ultimately leading to improved treatment options for patients.
Growing Investment in Rare Disease Research
Investment in rare disease research is witnessing a notable increase, significantly impacting the Rare Disease Treatment Market. In recent years, funding from both public and private sectors has surged, with venture capital investments in rare disease startups reaching USD 3 billion in 2023. This influx of capital is facilitating the exploration of novel treatment modalities and the advancement of clinical trials. As more resources are allocated to understanding and addressing rare diseases, the likelihood of discovering effective therapies increases, thereby enhancing market growth prospects.