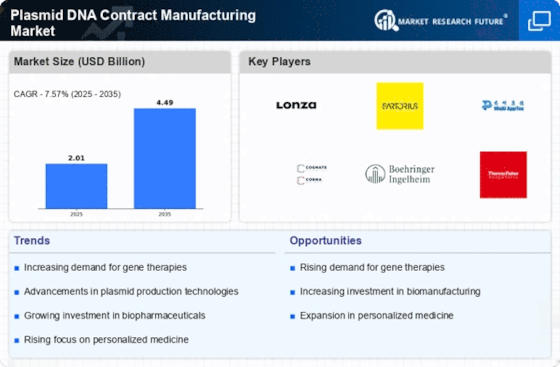
Growing Focus on Personalized Medicine
The shift towards personalized medicine is reshaping the landscape of the Plasmid DNA Contract Manufacturing Market. As healthcare moves towards tailored therapies, the demand for plasmid DNA that can be customized for individual patients is rising. This trend is particularly evident in gene therapies, where specific plasmid constructs are required to address unique genetic conditions. The market is likely to see a substantial increase in demand for contract manufacturing services that can accommodate these personalized approaches. Analysts predict that the market for personalized medicine will grow significantly, potentially reaching a valuation of over 200 billion in the next decade, thereby driving the need for specialized plasmid DNA manufacturing capabilities.
Emergence of New Therapeutic Applications
The emergence of new therapeutic applications is driving growth in the Plasmid DNA Contract Manufacturing Market. As research progresses, plasmid DNA is being explored for a variety of uses beyond traditional applications, including cancer immunotherapy and infectious disease prevention. This diversification of applications is expanding the market landscape, creating new opportunities for contract manufacturers. The increasing number of clinical trials focusing on innovative uses of plasmid DNA suggests a robust future demand. Market analysts estimate that the expansion into new therapeutic areas could lead to a 20% increase in the need for plasmid DNA manufacturing services over the next few years, as companies seek to capitalize on these emerging opportunities.
Increasing Investment in Biopharmaceuticals
Investment in biopharmaceuticals is a key driver for the Plasmid DNA Contract Manufacturing Market. As the biopharmaceutical sector expands, the need for high-quality plasmid DNA is becoming more pronounced. In recent years, funding for biopharmaceutical research has seen a notable increase, with venture capital and government grants supporting innovative projects. This influx of capital is expected to bolster the demand for contract manufacturing services, as companies seek reliable partners to produce plasmid DNA at scale. The market is anticipated to witness a significant uptick in demand, with estimates suggesting a potential increase of 15% in contract manufacturing services over the next few years, as biopharmaceutical companies prioritize efficiency and quality in their production processes.
Regulatory Support for Gene Therapy Products
Regulatory bodies are increasingly supportive of gene therapy products, which is positively impacting the Plasmid DNA Contract Manufacturing Market. Recent initiatives aimed at expediting the approval process for gene therapies have created a favorable environment for plasmid DNA manufacturers. This regulatory support not only encourages innovation but also instills confidence in investors and developers. As a result, the demand for plasmid DNA is expected to rise, with projections indicating a growth rate of around 10% annually in the contract manufacturing sector. The alignment of regulatory frameworks with industry needs is likely to facilitate smoother pathways for product development, further enhancing the attractiveness of plasmid DNA as a critical component in therapeutic solutions.
Technological Innovations in Plasmid DNA Production
The Plasmid DNA Contract Manufacturing Market is experiencing a surge in technological innovations that enhance production efficiency and quality. Advanced techniques such as high-throughput screening and automated bioreactors are being integrated into manufacturing processes. These innovations not only streamline operations but also reduce costs, making plasmid DNA more accessible for therapeutic applications. The market is projected to grow at a compound annual growth rate of approximately 12% over the next five years, driven by these advancements. Furthermore, the adoption of continuous manufacturing processes is likely to improve yield and consistency, which are critical for meeting the increasing demand for plasmid DNA in various applications, including vaccine development and gene therapy.