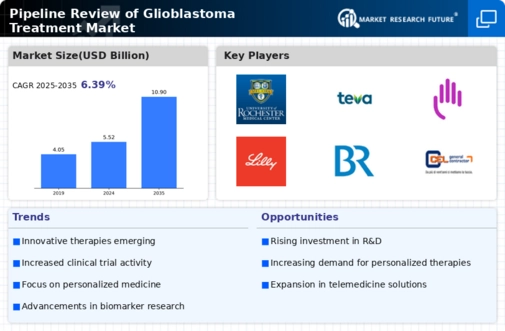
Increased R&D Investment
The Pipeline Review of Glioblastoma Treatment Market is witnessing a surge in research and development investment. Pharmaceutical companies are allocating substantial resources to discover novel therapeutic agents and treatment modalities. This trend is driven by the urgent need for effective glioblastoma therapies, as current treatment options often yield limited success. In 2025, it is estimated that R&D spending in oncology, particularly for glioblastoma, could reach unprecedented levels, potentially exceeding 20 billion USD. This influx of funding is likely to accelerate the development of innovative treatments, thereby enhancing the overall landscape of the Pipeline Review of Glioblastoma Treatment Market.
Growing Incidence of Glioblastoma
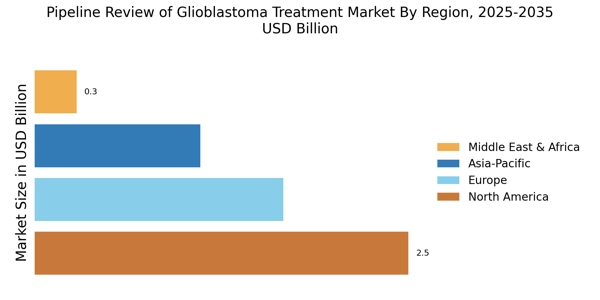
The Pipeline Review of Glioblastoma Treatment Market is significantly influenced by the rising incidence of glioblastoma. Epidemiological studies indicate that the global incidence of glioblastoma is increasing, with an estimated annual rate of 3.19 per 100,000 individuals. This alarming trend underscores the urgent need for effective treatment options. As the patient population grows, the demand for innovative therapies is likely to escalate, prompting pharmaceutical companies to prioritize glioblastoma in their pipelines. This increasing prevalence is a critical driver for the Pipeline Review of Glioblastoma Treatment Market, as it creates a pressing need for advancements in treatment.
Collaboration Between Academia and Industry
Collaboration between academic institutions and pharmaceutical companies is emerging as a vital driver in the Pipeline Review of Glioblastoma Treatment Market. These partnerships facilitate the exchange of knowledge, resources, and expertise, accelerating the development of innovative treatments. Academic research often provides foundational insights that can be translated into clinical applications, while industry partners bring the necessary funding and infrastructure. In recent years, numerous collaborations have been established, focusing on glioblastoma research. This synergy is likely to enhance the efficiency of drug development processes, ultimately benefiting the Pipeline Review of Glioblastoma Treatment Market by introducing novel therapies to the market more rapidly.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies in the Pipeline Review of Glioblastoma Treatment Market. Initiatives such as expedited review processes and breakthrough therapy designations are being implemented to facilitate the approval of promising treatments. This regulatory environment encourages pharmaceutical companies to invest in glioblastoma research, as the pathway to market is becoming more streamlined. In recent years, several therapies have received accelerated approval, indicating a shift towards a more favorable regulatory landscape. This trend is expected to continue, potentially leading to a higher number of effective treatments entering the Pipeline Review of Glioblastoma Treatment Market.
Technological Advancements in Drug Development
Technological advancements are playing a pivotal role in shaping the Pipeline Review of Glioblastoma Treatment Market. Innovations such as artificial intelligence and machine learning are being utilized to enhance drug discovery processes. These technologies enable researchers to analyze vast datasets, identify potential drug candidates, and predict treatment outcomes more efficiently. As a result, the time required to bring new therapies to market is likely to decrease. Furthermore, the integration of advanced imaging techniques is improving the ability to monitor treatment responses in glioblastoma patients. This technological evolution is expected to drive the Pipeline Review of Glioblastoma Treatment Market forward, fostering the development of more effective therapies.