Innovative Therapeutic Approaches
The emergence of innovative therapeutic approaches is transforming the Phelan McDermid Syndrome Market. Recent advancements in gene therapy and personalized medicine hold promise for addressing the underlying genetic causes of the syndrome. Companies are increasingly investing in research to develop targeted therapies that could improve patient outcomes. For instance, the development of CRISPR technology and other gene-editing tools may offer new avenues for treatment. As these therapies progress through clinical trials, they are likely to attract significant attention from investors and stakeholders, potentially leading to a surge in market growth. The introduction of novel treatment options could reshape the therapeutic landscape for Phelan McDermid Syndrome.
Increased Funding for Rare Disease Research
The Phelan McDermid Syndrome Market is benefiting from increased funding for rare disease research. Governments and private organizations are recognizing the need for more research into rare genetic disorders, including Phelan McDermid Syndrome. This influx of funding is likely to facilitate the development of new diagnostic tools and treatment options. For example, initiatives aimed at fostering collaboration between academic institutions and pharmaceutical companies are becoming more common. Such partnerships may accelerate the pace of research and innovation, ultimately leading to improved care for patients. The financial support directed towards rare diseases could significantly enhance the visibility and viability of the Phelan McDermid Syndrome Market.
Growing Patient Advocacy and Support Networks
The establishment of patient advocacy and support networks is a crucial driver in the Phelan McDermid Syndrome Market. These organizations play a vital role in raising awareness, providing resources, and supporting families affected by the syndrome. As these networks grow, they are likely to enhance the visibility of Phelan McDermid Syndrome, leading to increased demand for medical services and research funding. Furthermore, advocacy groups often collaborate with researchers and healthcare providers to promote clinical trials and new treatment options. This synergy between advocacy and research may foster a more robust market environment, ultimately benefiting patients and their families.
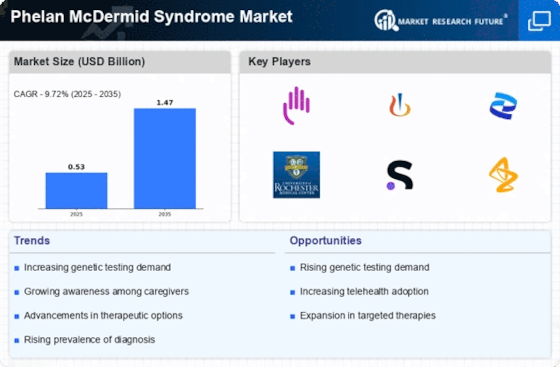
Rising Prevalence of Phelan McDermid Syndrome
The increasing prevalence of Phelan McDermid Syndrome is a notable driver in the Phelan McDermid Syndrome Market. Recent estimates suggest that the syndrome affects approximately 1 in 8,000 to 1 in 15,000 live births. This rising incidence is likely to lead to a greater demand for diagnostic tools and therapeutic interventions. As awareness grows among healthcare professionals and families, more cases are being identified, which could potentially expand the market for treatments and support services. The heightened focus on early diagnosis and intervention may also stimulate research and development efforts, thereby enhancing the overall landscape of the Phelan McDermid Syndrome Market.
Regulatory Support for Rare Disease Treatments
Regulatory support for rare disease treatments is emerging as a significant driver in the Phelan McDermid Syndrome Market. Regulatory agencies are increasingly implementing policies that expedite the approval process for therapies targeting rare conditions. This trend is likely to encourage pharmaceutical companies to invest in the development of treatments for Phelan McDermid Syndrome. The introduction of orphan drug designations and other incentives may facilitate faster access to innovative therapies for patients. As regulatory frameworks evolve to support rare disease research and development, the Phelan McDermid Syndrome Market could experience accelerated growth and enhanced treatment options for affected individuals.