Expansion of Healthcare Infrastructure
The Peripheral Guidewires Devices Market is benefiting from the expansion of healthcare infrastructure, particularly in emerging economies. As healthcare facilities improve and expand, there is a corresponding increase in the availability of advanced medical devices, including guidewires. This expansion is driven by rising healthcare expenditures and government initiatives aimed at improving access to quality healthcare. Consequently, the demand for peripheral guidewires is likely to increase as more healthcare providers adopt advanced technologies to enhance patient care. Recent reports indicate that healthcare spending in developing regions is projected to grow by over 10% annually, suggesting a robust market opportunity for peripheral guidewires in these expanding healthcare systems.
Regulatory Compliance and Quality Standards
The Peripheral Guidewires Devices Market is also shaped by stringent regulatory compliance and quality standards imposed by health authorities. These regulations ensure that guidewires meet safety and efficacy benchmarks, which is crucial for maintaining patient trust and ensuring successful outcomes. Manufacturers are increasingly investing in research and development to comply with these regulations, which can lead to enhanced product offerings. The focus on quality assurance is likely to foster innovation within the industry, as companies strive to develop guidewires that not only meet regulatory requirements but also exceed market expectations. This commitment to quality is expected to drive growth in the market as healthcare providers seek reliable and effective devices.
Rising Demand for Minimally Invasive Procedures
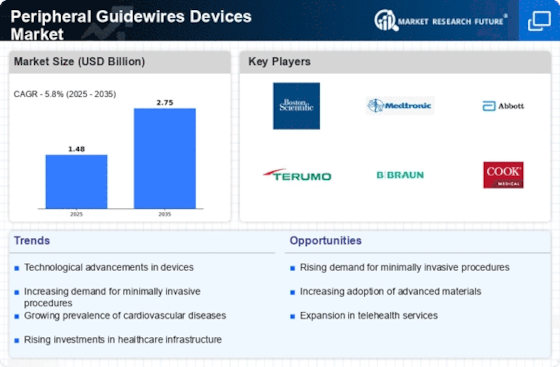
The Peripheral Guidewires Devices Market is significantly influenced by the increasing preference for minimally invasive surgical techniques. Patients and healthcare providers alike are gravitating towards procedures that promise reduced recovery times, lower risk of complications, and minimal scarring. Guidewires play a pivotal role in these procedures, facilitating access to targeted areas with precision. As a result, the market for peripheral guidewires is projected to expand, driven by the growing adoption of techniques such as endovascular surgery and catheter-based interventions. Recent statistics indicate that the minimally invasive surgery market is expected to reach USD 50 billion by 2026, highlighting the potential for peripheral guidewires to capture a substantial share of this burgeoning sector.
Increasing Prevalence of Cardiovascular Diseases
The rising incidence of cardiovascular diseases is a significant driver for the Peripheral Guidewires Devices Market. As the global population ages, the prevalence of conditions such as coronary artery disease and peripheral artery disease is escalating. This trend necessitates the use of guidewires in various interventional procedures, including angioplasty and stenting. Data suggests that cardiovascular diseases account for nearly 31% of all global deaths, underscoring the urgent need for effective treatment options. Consequently, the demand for peripheral guidewires is expected to rise, as healthcare providers seek to improve patient outcomes through minimally invasive techniques. This growing need for effective cardiovascular interventions is likely to propel the market forward.
Technological Advancements in Peripheral Guidewires Devices
The Peripheral Guidewires Devices Market is experiencing a surge in technological advancements, which are enhancing the efficacy and safety of medical procedures. Innovations such as hydrophilic coatings and advanced materials are improving the maneuverability and flexibility of guidewires. These developments are crucial as they allow for better navigation through complex vascular structures, thereby reducing procedural complications. Furthermore, the integration of digital technologies, such as imaging and navigation systems, is streamlining the use of guidewires in various interventions. According to recent data, the market for advanced guidewires is projected to grow at a compound annual growth rate of approximately 6.5% over the next few years, indicating a robust demand for these innovative solutions.