Supportive Regulatory Environment
A supportive regulatory environment is crucial for the growth of the On-body Injector Market. Regulatory bodies are increasingly recognizing the importance of innovative drug delivery systems and are streamlining approval processes for on-body injectors. This trend is evident in the recent initiatives aimed at expediting the review of new medical devices, which encourages manufacturers to invest in research and development. As a result, the On-body Injector Market is likely to benefit from a faster time-to-market for new products, fostering competition and innovation. Furthermore, favorable regulations can enhance patient access to advanced treatment options, thereby driving market growth.
Rising Prevalence of Chronic Diseases
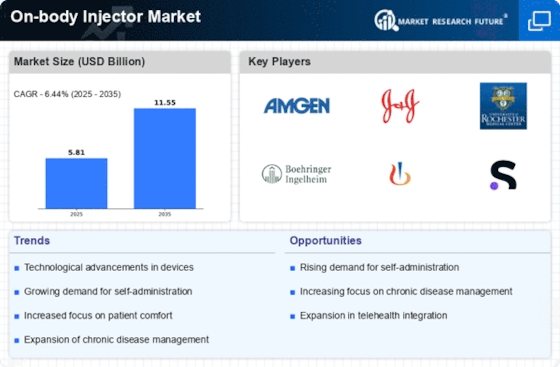
The On-body Injector Market is experiencing growth due to the increasing prevalence of chronic diseases such as diabetes, rheumatoid arthritis, and multiple sclerosis. As these conditions require regular medication administration, the demand for convenient and efficient drug delivery systems rises. According to recent data, the number of individuals diagnosed with diabetes is projected to reach 700 million by 2045, which underscores the need for innovative solutions like on-body injectors. These devices not only enhance patient compliance but also improve the overall quality of life for patients managing chronic conditions. Consequently, the On-body Injector Market is likely to expand as healthcare providers seek to offer more effective treatment options.
Increasing Demand for Self-Administration
The On-body Injector Market is witnessing a surge in demand for self-administration devices, driven by a growing preference among patients for autonomy in managing their health. Patients increasingly seek solutions that allow them to administer medications without the need for healthcare professional assistance. This trend is particularly evident in the treatment of chronic diseases, where regular injections are necessary. Market data suggests that the self-injection market is expected to grow at a CAGR of 10% over the next five years. As patients become more empowered and informed, the On-body Injector Market is likely to adapt by offering user-friendly devices that facilitate self-administration, thereby enhancing patient satisfaction and adherence.
Technological Innovations in Drug Delivery
Technological advancements play a pivotal role in shaping the On-body Injector Market. Innovations such as miniaturization, smart technology integration, and enhanced biocompatibility are driving the development of more sophisticated on-body injectors. For instance, the incorporation of sensors and connectivity features allows for real-time monitoring of medication adherence and patient health metrics. This trend is supported by data indicating that The On-body Injector is expected to reach USD 1.5 trillion by 2025, with on-body injectors representing a significant segment. As technology continues to evolve, the On-body Injector Market is poised for substantial growth, catering to the needs of both patients and healthcare providers.
Growing Focus on Patient-Centric Healthcare
The On-body Injector Market is significantly influenced by the growing emphasis on patient-centric healthcare. Healthcare providers are increasingly prioritizing patient experiences and outcomes, leading to a demand for devices that enhance comfort and convenience. On-body injectors align with this trend by offering pain-free and discreet medication delivery options. Market Research Future indicates that patient satisfaction is a key driver of treatment adherence, and devices that improve the overall experience are likely to see increased adoption. As the healthcare landscape evolves towards a more patient-centered approach, the On-body Injector Market is expected to thrive, catering to the needs and preferences of patients.