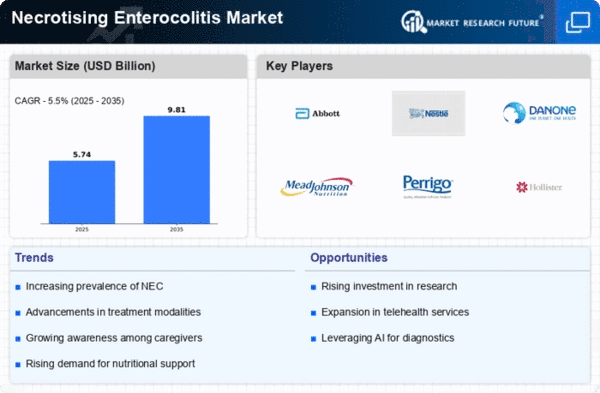
Top Industry Leaders in the Necrotising Enterocolitis Market

Latest Necrotising Enterocolitis Companies Updates
Jan 2024
BioMarin Pharmaceutical Received FDA Orphan Drug Designation for their investigational gene therapy program BMN-307, offering potential for a curative treatment for NEC.Partnered with leading research institutions to advance clinical trials and development of BMN-307.
Cytokinetics Presented positive Phase 2a data demonstrating efficacy and safety of their investigational drug CK-3505 in improving cardiac function in patients with NEC.Planned further clinical trials to confirm and expand upon these promising results.
Translarna Therapeutics Announced preclinical proof-of-concept data suggesting their investigational RNA therapy, ATL1102, could potentially improve cardiac function in models of NEC.Initiated pre-clinical safety and efficacy studies for ATL1102 to pave the way for clinical trials.
National Organization for Rare Disorders Launched a new awareness campaign focused on NEC, aiming to educate healthcare professionals and the public about the condition.Provided resources and support to patients and families affected by NEC.
List of Necrotising Enterocolitis Key companies in the market
- Abbott Laboratories Inc. (U.S.)
- Astellas Pharma Inc. (Japan)
- Bayer HealthCare AG (Germany)
- Becton, Dickinson, and Company (BD) (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Daiichi Sankyo Company, Ltd. (Japan)
- Eli Lilly and Co. (U.S.)
- Enzon Pharmaceuticals, Inc. (U.S.)
- Hoffmann-La Roche Ltd (Switzerland)
- GI Supply (U.S.)
- GlaxoSmithKline Plc (UK)