- Q2 2024: Pfizer Announces Collaboration with The LAM Foundation to Advance Research in Lymphangioleiomyomatosis Market Pfizer announced a strategic research collaboration with The LAM Foundation to support clinical research and accelerate the development of new therapies for lymphangioleiomyomatosis (LAM). The partnership aims to leverage Pfizer’s drug development expertise and The LAM Foundation’s patient network.
- Q1 2024: Novartis Initiates Phase II Clinical Trial of Everolimus for Lymphangioleiomyomatosis Market Novartis announced the initiation of a Phase II clinical trial evaluating the efficacy and safety of everolimus in patients with lymphangioleiomyomatosis (LAM), marking a significant step in expanding treatment options for this rare lung disease.
- Q2 2024: FDA Grants Orphan Drug Designation to Intas Pharmaceuticals’ LAM-101 for Lymphangioleiomyomatosis Market The U.S. Food and Drug Administration granted Orphan Drug Designation to Intas Pharmaceuticals’ investigational therapy LAM-101 for the treatment of lymphangioleiomyomatosis, providing incentives for further development.
- Q3 2024: Apotex Receives Health Canada Approval for Generic Sirolimus for Lymphangioleiomyomatosis Market Apotex announced Health Canada approval for its generic version of sirolimus, indicated for the treatment of lymphangioleiomyomatosis, expanding access to this important therapy for Canadian patients.
- Q2 2024: Zydus Pharmaceuticals Launches Sirolimus Tablets for Lymphangioleiomyomatosis Market in the U.S. Market Zydus Pharmaceuticals announced the U.S. launch of its sirolimus tablets, approved for the treatment of lymphangioleiomyomatosis, providing a new generic option for patients.
- Q1 2025: Taj Pharmaceuticals Expands Manufacturing Facility to Support LAM Drug Production Taj Pharmaceuticals announced the expansion of its manufacturing facility in India to increase production capacity for its lymphangioleiomyomatosis (LAM) drug portfolio, aiming to meet rising global demand.
- Q2 2025: Dr. Reddy’s Laboratories Receives EMA Approval for Sirolimus for Lymphangioleiomyomatosis Market Dr. Reddy’s Laboratories announced that the European Medicines Agency (EMA) has approved its sirolimus formulation for the treatment of lymphangioleiomyomatosis, enabling access to patients across Europe.
- Q3 2024: Terumo Corporation Announces Strategic Partnership with Morgan Scientific for LAM Diagnostic Devices Terumo Corporation entered into a strategic partnership with Morgan Scientific to co-develop and commercialize advanced diagnostic devices for early detection and monitoring of lymphangioleiomyomatosis.
- Q2 2024: Inogen Launches Portable Oxygen Concentrator for LAM Patients Inogen, Inc. announced the launch of a new portable oxygen concentrator specifically designed for patients with lymphangioleiomyomatosis, aiming to improve mobility and quality of life.
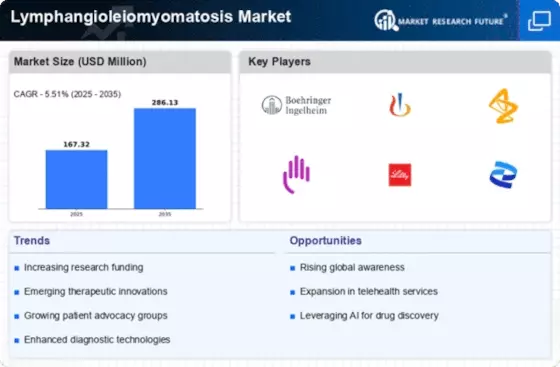
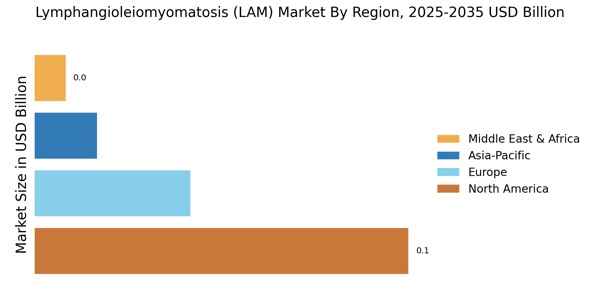
Lymphangioleiomyomatosis Market (LAM) Key Market Players & Competitive Insights
Leading market players are investing largely in the research and development to expand their product lines, which will help the Lymphangioleiomyomatosis Market (LAM) market grow even more. Market participants are also undertaking different strategic activities to spread their global footprint, with important market developments including new product launches, contractual agreements, higher investments, mergers and acquisitions, and collaboration with other organizations. To expand and sustain in a more competitive and rising market climate, Lymphangioleiomyomatosis Market (LAM) industry must give cost-effective items.
Manufacturing to minimize operational costs is one of the prime business tactics used by the manufacturers in the global Lymphangioleiomyomatosis Market (LAM) industry to benefit clients and increase the market sector. In recent years, the Lymphangioleiomyomatosis Market (LAM) industry has offered some of the major significant advantages to healthcare. Major players in the Lymphangioleiomyomatosis Market (LAM) market, including Pfizer Inc., Intas Pharmaceuticals Ltd, Apotex Inc., Novartis AG, Zydus Pharmaceuticals, Inc., Taj Pharmaceuticals Limited, Morgan Scientific Inc., Dr. Reddy’s Laboratories Ltd, Terumo Corporation, Inogen, Inc., and others, are trying to raise market demand by investing in the research and the development operations.
Novartis AG, an international pharmaceutical company, is known for its dedication to advancing healthcare through innovative research and development. Founded in 1996, Novartis has since established itself as a leader in the pharmaceutical industry, with a diverse portfolio of prescription medicines, vaccines, and consumer health products. The company operates through three main business segments: Innovative Medicines, Sandoz (generics), and Alcon (eye care).
Novartis is renowned for its groundbreaking work in developing treatments for a wider range of diseases, including oncology, cardiovascular diseases, respiratory conditions, and neurological disorders. In May 2021, Novartis AG launched EMPATHY with Molecular Partners, clinical trials for ensovibep, potentially increasing treatment options for lymphangioleiomyomatosis (LAM) patients. This focuses on improving Novartis' portfolio and boosting market growth through timely patient interventions and therapeutic advancements.
Pfizer Inc. is an international pharmaceutical company headquartered in New York City, United States. Established in 1849, Pfizer has grown into one of the world's largest and most renowned pharmaceutical companies, with a diverse portfolio of prescription medications, vaccines, and consumer healthcare products. The company operates across various therapeutic areas, including oncology, cardiology, immunology, and vaccines, and is known for its innovative research and development efforts. Pfizer has brought to market numerous blockbuster drugs, such as Viagra, Lipitor, and Prevnar, which have significantly impacted public health worldwide. In April 2021, Pfizer Inc. acquired Amplyx Pharmaceuticals, enriching its anti-infectives pipeline.
Key Companies in the Lymphangioleiomyomatosis Market (LAM) market include
- Pfizer Inc.
- Intas Pharmaceuticals Ltd
- Apotex Inc.
- Novartis AG
- Zydus Pharmaceuticals, Inc.
- Taj Pharmaceuticals Limited
- Morgan Scientific Inc.
- Reddy’s Laboratories Ltd
- Terumo Corporation
- Inogen, Inc.