Expansion of Clinical Applications
The real time-pcr-qpcr market is witnessing an expansion of clinical applications, which is driving its growth in Japan. The versatility of real time-pcr-qpcr technologies allows for their use in various fields, including oncology, infectious diseases, and genetic disorders. As healthcare professionals increasingly recognize the potential of these technologies, the demand for real time-pcr-qpcr systems is expected to rise. In 2025, the oncology segment alone is projected to account for over 30% of the total market share, reflecting a growing reliance on molecular diagnostics for cancer detection and monitoring. This diversification of applications not only enhances the market's resilience but also encourages further innovation in real time-pcr-qpcr technologies, ultimately benefiting patient outcomes and healthcare efficiency.
Growing Awareness of Infectious Diseases
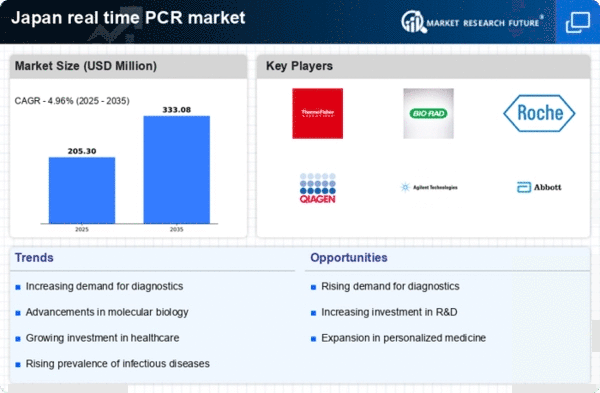
In Japan, there is an increasing awareness of infectious diseases, which is significantly impacting the real time-pcr-qpcr market. The population's heightened concern regarding health issues has led to a greater demand for rapid and accurate diagnostic tools. As a result, healthcare facilities are investing in advanced real time-pcr-qpcr systems to enhance their testing capabilities. According to recent data, the market for infectious disease diagnostics is expected to grow at a CAGR of 10% through 2027. This growth is indicative of the rising need for effective disease management strategies, which rely heavily on the precision offered by real time-pcr-qpcr technologies. Consequently, the real time-pcr-qpcr market is likely to benefit from this trend, as healthcare providers seek to implement more robust diagnostic solutions.
Rising Research and Development Investments
The real time-pcr-qpcr market in Japan is experiencing a surge in research and development investments. This trend is driven by both public and private sectors aiming to enhance diagnostic capabilities and therapeutic solutions. In 2025, R&D spending in the biotechnology sector is projected to reach approximately ¥1 trillion, reflecting a 15% increase from previous years. Such investments are likely to foster innovation in real time-pcr-qpcr technologies, leading to more efficient and accurate testing methods. Furthermore, collaborations between academic institutions and industry players are becoming more prevalent, facilitating the development of novel applications in infectious disease detection and genetic analysis. This collaborative environment is expected to propel the growth of the real time-pcr-qpcr market, as new technologies emerge to meet the evolving needs of healthcare providers and researchers alike.
Technological Integration with Digital Health
The integration of real time-pcr-qpcr technologies with digital health solutions is emerging as a key driver in Japan. As healthcare systems increasingly adopt digital platforms for data management and patient monitoring, the synergy between these technologies is becoming apparent. Real time-pcr-qpcr systems can now be seamlessly integrated with electronic health records and telemedicine applications, facilitating real-time data sharing and analysis. This integration is expected to enhance the efficiency of diagnostic processes and improve patient care. In 2025, the digital health market in Japan is anticipated to reach ¥500 billion, indicating a strong potential for collaboration with the real time-pcr-qpcr market. Such advancements may lead to more personalized healthcare solutions, ultimately driving the growth of the real time-pcr-qpcr market.
Increased Government Funding for Health Initiatives
In Japan, increased government funding for health initiatives is playing a crucial role in the growth of the real time-pcr-qpcr market. The government has recognized the importance of advanced diagnostic technologies in improving public health outcomes. In 2025, it is estimated that public health funding will increase by 20%, with a significant portion allocated to enhancing laboratory infrastructure and diagnostic capabilities. This funding is likely to support the acquisition of state-of-the-art real time-pcr-qpcr systems, enabling healthcare facilities to provide timely and accurate testing services. Additionally, government initiatives aimed at promoting research and development in molecular diagnostics are expected to further stimulate the market. As a result, the real time-pcr-qpcr market is poised for substantial growth, driven by these supportive policies and investments.
















Leave a Comment