Market Trends
Key Emerging Trends in the IV Fluid Monitoring Devices Market
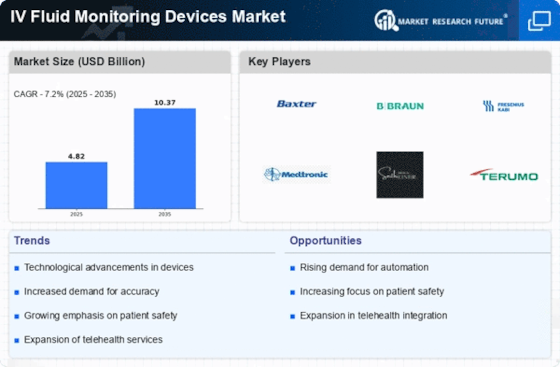
The IV fluid monitoring devices market is experiencing a surge in demand due to the increasing prevalence of chronic diseases, rising surgical procedures, and the growing elderly population, leading to higher demand for accurate fluid management. Becoming it real-life, technology changes, like sensors, the Internet of things, and real-time examination enhancement, are pressurizing the precision, and efficiency of IV fluid monitoring devices, making market quickly growing. The introduction of home tele-nursing, wherein remote monitoring solutions for IV fluid administering are performed through wearable devices and online platforms, is enhancing the quality of care offered leading to high growth of market particularly in home care settings. A sharp healthcare focus on patient safety and the reduction of medication errors has led hospitals to invest in IV fluid monitoring devices that enable the ultimate product delivery into the vein and the avoidance of adverse events, which contributes to the faster market growth. IV fluid monitoring devices have an option to link to electronic health record systems which facilitates secure data transmission and documentation without workflow disruption. Electronic health records (EHR) make real-time data integration possible which in turn makes the market hugely promising. The introduction of stringent regulatory specifications for medical devices, including IV fluid monitoring systems, in turn plays a critical role in changing market mechanics and fostering innovation that is primarily focused on the compliance with ever growing, tight regulatory requirements, consequently influencing the market growth. Among the principal advantages of IV fluid monitoring features incorporated in smart infusion pumps lifting patient follow up up to the current state of instant infusion rate adjustment if patient’s parameters change and IV fluid monitoring equipment thereby improve outcomes and drive the demand. Healthcare providers continue to be on a mission to seek the most economical options of IV fluid monitoring devices that can be sold in the market at competitive rates, have value features and help in cost reducing. The adoption of a patient-centric approach in healthcare delivery, with a focus on personalized treatment and improved patient outcomes, is driving market demand for IV fluid monitoring devices that offer tailored solutions for individual patient needs.


















Leave a Comment