

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
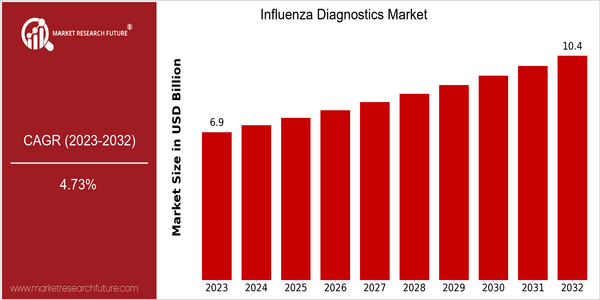
Market Size Snapshot
| Year | Value |
|---|---|
| 2023 | USD 6.86 Billion |
| 2032 | USD 10.4 Billion |
| CAGR (2024-2032) | 4.73 % |
Note – Market size depicts the revenue generated over the financial year
The global Influenza Diagnostics Market is currently valued at approximately USD 6.86 billion in 2023 and is projected to reach USD 10.4 billion by 2032, reflecting a compound annual growth rate (CAGR) of 4.73% from 2024 to 2032. This growth trajectory indicates a steady increase in demand for influenza diagnostic solutions, driven by rising incidences of influenza outbreaks and the growing awareness of the importance of early diagnosis in managing viral infections. The market's expansion is further supported by advancements in diagnostic technologies, including the development of rapid testing kits and molecular diagnostics, which enhance the accuracy and speed of influenza detection. Key factors propelling this market include the increasing prevalence of influenza viruses, particularly in the wake of global health challenges, and the ongoing investments in healthcare infrastructure aimed at improving disease surveillance and response capabilities. Notable companies such as Roche Diagnostics, Abbott Laboratories, and Thermo Fisher Scientific are at the forefront of innovation in this sector, actively engaging in strategic partnerships and product launches to enhance their diagnostic offerings. For instance, recent collaborations focused on integrating artificial intelligence with diagnostic tools are expected to further streamline testing processes and improve patient outcomes, thereby contributing to the overall growth of the influenza diagnostics market.
Regional Market Size
Regional Deep Dive
The Influenza Diagnostics Market is characterized by a growing demand for rapid and accurate diagnostic tools across various regions. In North America, the market is driven by advanced healthcare infrastructure, high prevalence of influenza, and significant investments in research and development. Europe showcases a strong regulatory framework and increasing awareness about influenza prevention, while Asia-Pacific is witnessing a surge in healthcare spending and technological advancements. The Middle East and Africa face unique challenges, including limited access to healthcare resources, but are gradually adopting innovative diagnostic solutions. Latin America is experiencing growth due to rising healthcare initiatives and increasing public awareness about influenza.
Europe
- The European Centre for Disease Prevention and Control (ECDC) has launched initiatives to promote the use of rapid influenza diagnostics, aiming to improve response times during flu seasons.
- Innovations in molecular diagnostics, such as PCR-based tests, are gaining traction in Europe, driven by companies like QIAGEN and bioMérieux, which are focusing on enhancing test sensitivity and specificity.
Asia Pacific
- Countries like Japan and Australia are leading the way in adopting advanced influenza diagnostic technologies, with government support for public health initiatives aimed at controlling outbreaks.
- The rise of telemedicine in the region is facilitating remote diagnostics and consultations, allowing for quicker identification and management of influenza cases.
Latin America
- The Pan American Health Organization (PAHO) has been instrumental in promoting influenza vaccination and diagnostics, leading to increased public health awareness and testing capabilities.
- Emerging companies in Brazil and Mexico are focusing on developing affordable diagnostic kits, which are expected to enhance access to influenza testing in underserved areas.
North America
- The U.S. Food and Drug Administration (FDA) has recently approved several new rapid influenza diagnostic tests, enhancing the speed and accuracy of influenza detection in clinical settings.
- Key players like Abbott Laboratories and Roche Diagnostics are investing heavily in R&D to develop next-generation influenza diagnostics, which are expected to improve patient outcomes and reduce healthcare costs.
Middle East And Africa
- The World Health Organization (WHO) is actively working with governments in the region to improve influenza surveillance and diagnostics, which is crucial for managing seasonal outbreaks.
- Local companies are beginning to develop cost-effective diagnostic solutions tailored to the unique healthcare challenges faced in the region, such as limited access to advanced technologies.
Did You Know?
“Approximately 3 to 5 million cases of severe influenza occur globally each year, leading to 290,000 to 650,000 respiratory deaths, highlighting the critical need for effective diagnostic tools.” — World Health Organization (WHO)
Segmental Market Size
The Influenza Diagnostics Market is currently experiencing stable growth, driven by increasing awareness of influenza's impact on public health and the need for rapid diagnosis. Key factors propelling demand include the rising incidence of influenza outbreaks and advancements in diagnostic technologies, such as molecular assays and point-of-care testing. Regulatory policies promoting early detection and treatment further enhance market dynamics, ensuring timely intervention during flu seasons. Currently, the market is in a mature adoption stage, with companies like Roche and Abbott leading in the deployment of innovative diagnostic solutions. Primary applications include hospital settings, outpatient clinics, and home testing kits, where rapid and accurate diagnosis is crucial for effective patient management. Trends such as the ongoing threat of pandemics and government mandates for vaccination and testing are catalyzing growth. Technologies like CRISPR-based diagnostics and next-generation sequencing are shaping the segment's evolution, enabling more precise and efficient influenza detection methods.
Future Outlook
The Influenza Diagnostics Market is poised for significant growth from 2023 to 2032, with a projected market value increase from $6.86 billion to $10.4 billion, reflecting a compound annual growth rate (CAGR) of 4.73%. This growth trajectory is driven by an increasing prevalence of influenza infections, heightened awareness of respiratory diseases, and advancements in diagnostic technologies. As healthcare systems worldwide continue to prioritize rapid and accurate diagnostic solutions, the demand for innovative influenza testing methods, including molecular diagnostics and point-of-care testing, is expected to rise substantially. By 2032, it is anticipated that the penetration of rapid diagnostic tests will reach approximately 60% of the market, significantly enhancing the speed of diagnosis and treatment initiation. Key technological drivers such as the integration of artificial intelligence in diagnostic tools and the development of multiplex assays will further shape the market landscape. These innovations not only improve the accuracy and efficiency of influenza diagnostics but also facilitate the simultaneous detection of multiple respiratory pathogens, catering to the growing need for comprehensive testing solutions. Additionally, supportive government policies and funding for influenza surveillance and vaccination programs are likely to bolster market growth. Emerging trends, including the increasing adoption of telemedicine and home testing kits, will also play a crucial role in expanding access to influenza diagnostics, ultimately contributing to improved public health outcomes and a more resilient healthcare infrastructure.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 5.3% |
Influenza Diagnostics Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









