Regulatory Support for Orphan Drugs
Regulatory support for orphan drugs is a significant driver for the Infantile Systemic Hyalinosis Market. Governments around the world are implementing policies that incentivize the development of treatments for rare diseases, including Infantile Systemic Hyalinosis Market. These incentives may include tax breaks, extended market exclusivity, and expedited review processes for orphan drugs. Such regulatory frameworks encourage pharmaceutical companies to invest in the development of therapies for conditions that may otherwise be overlooked due to limited market potential. As a result, the Infantile Systemic Hyalinosis Market is likely to benefit from an increase in the number of available treatment options, ultimately improving patient outcomes.
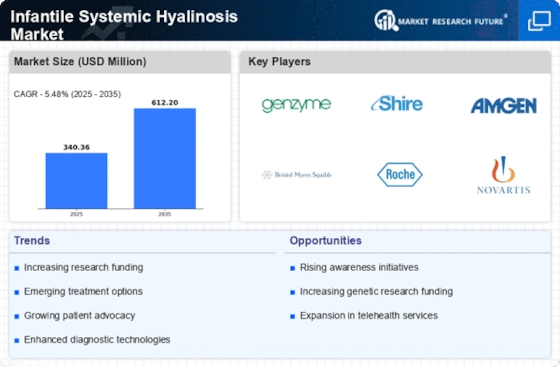
Rising Incidence of Rare Genetic Disorders
The increasing incidence of rare genetic disorders, including Infantile Systemic Hyalinosis Market, is a notable driver for the Infantile Systemic Hyalinosis Market. As awareness of these conditions grows, healthcare providers are more likely to diagnose and report cases, leading to a higher demand for treatment options. Recent estimates suggest that the prevalence of rare diseases affects approximately 1 in 15 individuals, which translates to a significant number of potential patients for the Infantile Systemic Hyalinosis Market. This rising incidence not only highlights the need for effective therapies but also encourages pharmaceutical companies to invest in research and development, thereby expanding the market landscape.
Growing Investment in Rare Disease Research
Growing investment in rare disease research is a critical driver for the Infantile Systemic Hyalinosis Market. Governments and private organizations are increasingly allocating funds to support research initiatives aimed at understanding and treating rare genetic disorders. This influx of capital is likely to accelerate the development of innovative therapies and clinical trials specifically targeting Infantile Systemic Hyalinosis Market. According to recent reports, funding for rare disease research has seen a substantial increase, with billions of dollars being invested annually. This trend not only fosters collaboration among researchers and pharmaceutical companies but also enhances the overall market potential for the Infantile Systemic Hyalinosis Market.
Increased Patient Advocacy and Support Groups
Increased patient advocacy and support groups play a pivotal role in shaping the Infantile Systemic Hyalinosis Market. These organizations raise awareness about the condition, educate patients and families, and advocate for better access to treatments. Their efforts contribute to a more informed patient population, which can lead to higher rates of diagnosis and treatment adherence. Furthermore, patient advocacy groups often collaborate with pharmaceutical companies to facilitate clinical trials and research initiatives, thereby driving innovation within the Infantile Systemic Hyalinosis Market. The growing presence of these organizations is likely to enhance the visibility of the condition and promote the development of new therapies.
Technological Advancements in Diagnostic Tools
Technological advancements in diagnostic tools are transforming the landscape of the Infantile Systemic Hyalinosis Market. Enhanced genetic testing methods, such as next-generation sequencing, allow for earlier and more accurate diagnosis of this rare condition. The ability to identify genetic mutations associated with Infantile Systemic Hyalinosis Market has led to improved patient outcomes and increased treatment options. As diagnostic technologies continue to evolve, the market is likely to see a surge in demand for targeted therapies, which could significantly impact the Infantile Systemic Hyalinosis Market. Furthermore, the integration of artificial intelligence in diagnostics may streamline the identification process, making it more efficient and accessible.