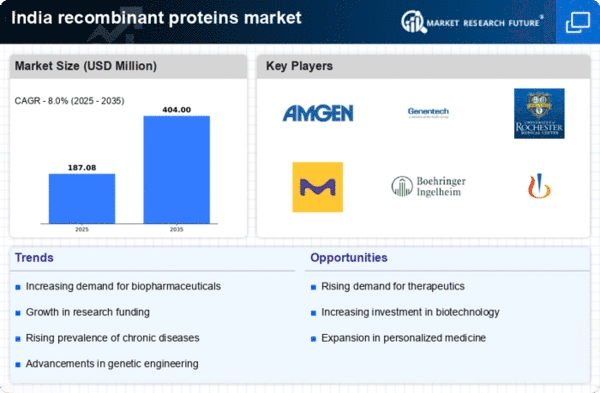
Advancements in Genetic Engineering
Technological innovations in genetic engineering are significantly impacting the recombinant proteins market. Techniques such as CRISPR and synthetic biology are enabling researchers to create more efficient and effective recombinant proteins. These advancements not only enhance the quality of proteins produced but also reduce production costs, making therapies more accessible. As a result, the market is likely to see an increase in the variety of recombinant proteins available for therapeutic use. The integration of these technologies could potentially lead to a market expansion of around 20% over the next few years, as companies leverage these tools to innovate and improve their product offerings.
Growing Prevalence of Chronic Diseases
The rising incidence of chronic diseases in India is driving the demand for therapeutic solutions, including recombinant proteins. Conditions such as diabetes, cancer, and autoimmune disorders are becoming increasingly common, necessitating effective treatment options. The recombinant proteins market is likely to benefit from this trend. These proteins play a crucial role in developing targeted therapies. According to recent estimates, the market for therapeutic proteins is projected to reach approximately $5 billion by 2026, reflecting a compound annual growth rate (CAGR) of 12%. This growing health crisis underscores the importance of recombinant proteins in addressing public health challenges.
Increasing Investment in Biotechnology
The recombinant proteins market in India is experiencing a surge in investment, particularly from both public and private sectors. Government initiatives aimed at promoting biotechnology research and development are likely to enhance the capabilities of local firms. For instance, the Biotechnology Industry Research Assistance Council (BIRAC) has been instrumental in funding innovative projects, which could lead to the development of novel recombinant proteins. This influx of capital is expected to bolster the market, potentially leading to a growth rate of around 15% annually. As companies expand their research facilities and production capabilities, the recombinant proteins market is expected to experience significant advancements.
Regulatory Support for Biopharmaceuticals
The regulatory landscape in India is evolving to support the growth of the recombinant proteins market. The Central Drugs Standard Control Organization (CDSCO) has been streamlining approval processes for biopharmaceuticals, which may encourage more companies to enter the market. This regulatory support is crucial for fostering innovation and ensuring that new recombinant proteins can reach the market more swiftly. As the approval timelines shorten, it is anticipated that the market could witness a growth rate of approximately 10% annually. This favorable environment is likely to attract both domestic and international players, further enhancing competition and innovation.
Rising Awareness and Acceptance of Biologics
There is a growing awareness and acceptance of biologics among healthcare professionals and patients in India. As education about the benefits of recombinant proteins increases, more healthcare providers are likely to prescribe these therapies. This shift in perception is crucial for the recombinant proteins market, as it could lead to higher adoption rates. Market Research Future indicates that the acceptance of biologics could increase by 25% over the next few years, driven by successful case studies and patient testimonials. This trend suggests a promising future for the recombinant proteins market, as it aligns with the broader movement towards personalized medicine.