Increasing Cancer Incidence
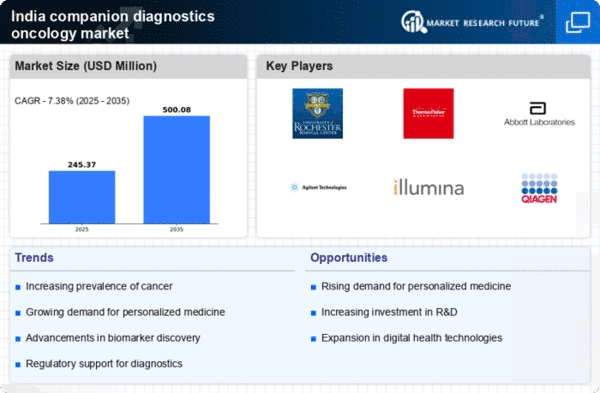
The rising incidence of cancer in India is a critical driver for the companion diagnostics-oncology market. According to the National Cancer Registry Programme, cancer cases are projected to increase significantly, with an estimated 1.5 million new cases expected by 2025. This alarming trend necessitates the development of targeted therapies, which rely heavily on companion diagnostics to identify suitable patients for specific treatments. As healthcare providers seek to improve patient outcomes, the demand for companion diagnostics is likely to grow, thereby enhancing the market's potential. The increasing awareness of cancer screening and early detection further fuels this growth, as patients and healthcare professionals alike recognize the importance of personalized treatment approaches in oncology.
Government Initiatives and Funding
Government initiatives aimed at enhancing cancer care in India are significantly impacting the companion diagnostics-oncology market. The Indian government has launched various programs to improve cancer treatment accessibility and affordability, including the National Health Mission and the Ayushman Bharat scheme. These initiatives often include funding for research and development in oncology, which encompasses companion diagnostics. With an estimated budget allocation of over $1 billion for cancer care in the next fiscal year, the government is likely to stimulate innovation in this sector. This financial support not only encourages the development of new diagnostic tools but also promotes collaborations between public and private entities, thereby fostering growth in the companion diagnostics-oncology market.
Rising Awareness of Precision Medicine
The growing awareness of precision medicine among healthcare professionals and patients is a significant driver for the companion diagnostics-oncology market. Educational campaigns and seminars are increasingly highlighting the benefits of personalized treatment approaches, which rely on companion diagnostics to identify the most effective therapies for individual patients. As a result, oncologists are more inclined to utilize these diagnostic tools in their practice, leading to improved patient outcomes. Market Research Future indicates that the adoption rate of precision medicine in oncology is expected to reach 30% by 2026 in India. This shift towards personalized care is likely to enhance the demand for companion diagnostics, as healthcare providers seek to optimize treatment strategies based on genetic and molecular profiling.
Technological Advancements in Diagnostics
Technological innovations in the field of diagnostics are propelling the companion diagnostics-oncology market forward. Advances in genomic sequencing, biomarker identification, and data analytics have made it possible to develop more precise diagnostic tools. For instance, next-generation sequencing (NGS) technologies are becoming increasingly accessible, allowing for comprehensive genomic profiling of tumors. This enables oncologists to tailor treatment plans based on individual patient profiles, thus improving therapeutic efficacy. The Indian market is witnessing a surge in the adoption of these technologies, with a projected growth rate of 15% annually. As diagnostic capabilities continue to evolve, the companion diagnostics-oncology market is expected to expand, driven by the need for more effective cancer management solutions.
Collaborative Research and Development Efforts
Collaborative research initiatives between academic institutions, healthcare providers, and biotechnology companies are fostering innovation in the companion diagnostics-oncology market. These partnerships aim to accelerate the development of new diagnostic tests and therapies tailored to specific cancer types. For instance, several Indian universities are collaborating with pharmaceutical companies to explore novel biomarkers that can enhance the accuracy of companion diagnostics. Such collaborations not only facilitate knowledge sharing but also provide access to funding and resources necessary for research. As these partnerships continue to grow, they are likely to drive advancements in the companion diagnostics-oncology market, ultimately leading to more effective cancer treatment options for patients.