Regulatory Incentives for Drug Development
Regulatory incentives for drug development are increasingly influencing the Hurler Scheie Syndrome Market. Governments and regulatory bodies are implementing policies to expedite the approval process for treatments targeting rare diseases. These incentives, such as orphan drug designations and fast-track approvals, encourage pharmaceutical companies to invest in the development of therapies for Hurler Scheie Syndrome Market. The potential for market exclusivity and financial benefits associated with these designations can significantly enhance the attractiveness of developing new treatments. As a result, the regulatory environment is likely to foster innovation and increase the number of available therapies for patients suffering from this rare genetic disorder.
Growing Investment in Rare Disease Research
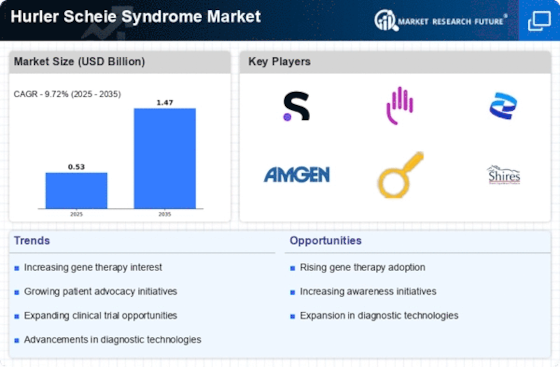
The growing investment in rare disease research is a crucial driver for the Hurler Scheie Syndrome Market. Pharmaceutical companies and research institutions are increasingly allocating resources to understand and develop treatments for rare genetic disorders. This trend is reflected in the substantial funding directed towards clinical trials and research initiatives aimed at Hurler Scheie Syndrome Market. In recent years, funding for rare disease research has surged, with estimates indicating that over 30% of new drug approvals are for rare diseases. This influx of investment not only accelerates the development of novel therapies but also fosters collaboration between academia and industry, ultimately benefiting patients with Hurler Scheie Syndrome Market.
Rising Prevalence of Hurler Scheie Syndrome
The increasing prevalence of Hurler Scheie Syndrome Market appears to be a significant driver for the Hurler Scheie Syndrome Market. As awareness of rare genetic disorders grows, more cases are being diagnosed, leading to a heightened demand for effective treatment options. Recent estimates suggest that the incidence of Hurler Scheie Syndrome Market is approximately 1 in 100,000 live births, which, while rare, indicates a consistent need for specialized medical care. This rising prevalence not only emphasizes the necessity for innovative therapies but also encourages pharmaceutical companies to invest in research and development. Consequently, the market is likely to witness an influx of new treatment modalities aimed at addressing the unique challenges posed by this condition.
Increased Patient Advocacy and Support Groups
Increased patient advocacy and support groups play a pivotal role in shaping the Hurler Scheie Syndrome Market. These organizations raise awareness about the condition, providing essential resources and support for affected families. Their efforts contribute to improved diagnosis rates and encourage research funding by highlighting the unmet needs of patients. Advocacy groups often collaborate with healthcare professionals and policymakers to promote access to treatments and clinical trials. As these organizations continue to grow in influence, they are likely to drive demand for innovative therapies and support services, thereby enhancing the overall market landscape for Hurler Scheie Syndrome Market.
Technological Advancements in Treatment Options
Technological advancements in treatment options are poised to transform the Hurler Scheie Syndrome Market. Innovations in gene therapy and enzyme replacement therapy have shown promise in addressing the underlying causes of the syndrome. For instance, recent developments in recombinant enzyme therapies have demonstrated efficacy in improving patient outcomes. The market for enzyme replacement therapies is projected to grow significantly, with estimates indicating a compound annual growth rate of over 10% in the coming years. These advancements not only enhance the quality of life for patients but also attract investment from biotechnology firms, thereby expanding the market landscape. As new therapies emerge, the industry is likely to experience increased competition and a broader array of treatment choices for patients.