Growing Awareness and Education
The growing awareness and education surrounding respiratory syncytial virus (RSV) are pivotal in shaping the Human Respiratory Syncytial Virus Treatment Market. Increased public and healthcare professional awareness about the symptoms, transmission, and potential complications of RSV is leading to earlier diagnosis and treatment. Educational campaigns aimed at parents and caregivers are particularly crucial, as they help in recognizing the signs of RSV in infants and young children. This heightened awareness is likely to result in more patients seeking medical attention, thereby increasing the demand for effective treatments. Furthermore, healthcare providers are becoming more proactive in screening and managing RSV cases, which could further stimulate the Human Respiratory Syncytial Virus Treatment Market as treatment options become more widely utilized.
Increased Healthcare Expenditure
Increased healthcare expenditure is a crucial factor driving the Human Respiratory Syncytial Virus Treatment Market. As nations allocate more resources to healthcare, there is a corresponding rise in funding for research, development, and treatment of infectious diseases, including respiratory syncytial virus (RSV). This trend is particularly evident in countries with aging populations, where the burden of RSV is more pronounced. Enhanced healthcare budgets facilitate the development of new therapies and improve access to existing treatments, thereby addressing the needs of affected populations. Furthermore, as healthcare systems evolve to meet the challenges posed by RSV, investments in preventive measures and treatment options are likely to increase. This growing financial commitment to healthcare is expected to bolster the Human Respiratory Syncytial Virus Treatment Market, ultimately benefiting patients and healthcare providers alike.
Advancements in Therapeutic Options
Recent advancements in therapeutic options for treating respiratory syncytial virus (RSV) are significantly influencing the Human Respiratory Syncytial Virus Treatment Market. The introduction of monoclonal antibodies and antiviral agents has transformed the treatment landscape, offering new hope for patients. For instance, palivizumab, a monoclonal antibody, has been widely used for the prevention of severe RSV disease in high-risk infants. Moreover, ongoing clinical trials are exploring novel antiviral compounds that may provide more effective treatment alternatives. As these innovative therapies gain regulatory approval and enter the market, they are expected to enhance treatment outcomes and increase patient access to care. This evolution in therapeutic options is likely to drive the Human Respiratory Syncytial Virus Treatment Market forward, as healthcare providers seek to adopt the latest advancements in RSV management.
Regulatory Support for Innovative Treatments
Regulatory support for innovative treatments is a significant driver of the Human Respiratory Syncytial Virus Treatment Market. Regulatory agencies are increasingly prioritizing the approval of novel therapies aimed at addressing unmet medical needs in RSV management. Fast-track designations and priority review processes are being granted to promising candidates, expediting their entry into the market. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, fostering innovation in the treatment landscape. As a result, the Human Respiratory Syncytial Virus Treatment Market is likely to experience accelerated growth, with new therapies becoming available to patients more rapidly than in the past. This trend not only enhances treatment options but also improves patient outcomes in managing RSV.
Rising Incidence of Respiratory Syncytial Virus
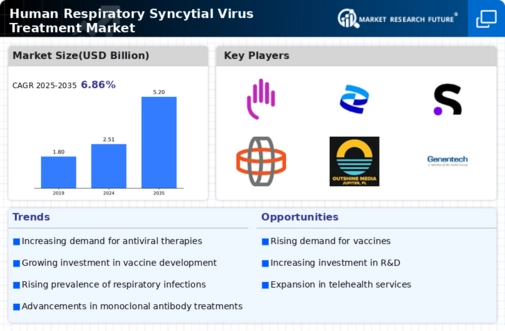
The increasing incidence of respiratory syncytial virus (RSV) infections is a primary driver for the Human Respiratory Syncytial Virus Treatment Market. Recent data indicates that RSV is responsible for a significant number of hospitalizations among infants and the elderly, with estimates suggesting that it leads to approximately 160,000 hospitalizations annually in the United States alone. This rising burden of disease necessitates the development and availability of effective treatment options, thereby propelling market growth. As healthcare systems grapple with the challenges posed by RSV, the demand for innovative therapies and preventive measures is likely to escalate. Consequently, pharmaceutical companies are investing in research and development to address this urgent public health issue, further stimulating the Human Respiratory Syncytial Virus Treatment Market.