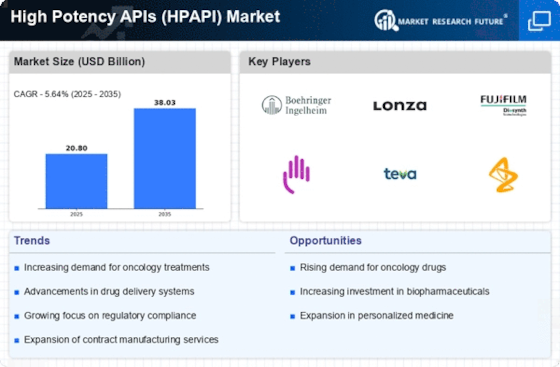
Top Industry Leaders in the High Potency APIs Market

Latest High Potency APIs Companies Updates
Beta Bionics iLet Bionic Pancreas: In May 2023, the FDA cleared the Beta Bionics iLet Bionic Pancreas system for individuals with type 1 diabetes aged 6 and above. This system combines the iLet insulin pump with the iLet Dosing Decision software, offering automated insulin delivery and improved glycemic control.
Medtronic MiniMed 780G: In April 2023, the MiniMed 780G system received FDA approval for use in individuals aged 7 and above. This advanced hybrid closed-loop system features automatic adjustments and real-time corrections to blood sugar levels, including automatic suspension of insulin delivery when glucose falls below a preset threshold.
Tandem Diabetes Control-IQ Technology: In January 2024, Tandem Diabetes received FDA approval for its Control-IQ technology with Basal-IQ and Auto Bolus features. This update to their existing t:slim X2 insulin pump system further automates insulin delivery for adults with type 1 diabetes.
List of High Potency APIs Key companies in the market:
- Teva Pharmaceuticals (Israel)
- Lilly (US)
- Pfizer Inc. (US)
- Carbogen Amcis (India)
- Bristol-Myers Squibb (US)
- Asymchem Lab (China)
- Lonza Group (Switzerland)
- Bayer AG (Germany)
- Boehringer Ingelheim (Germany)