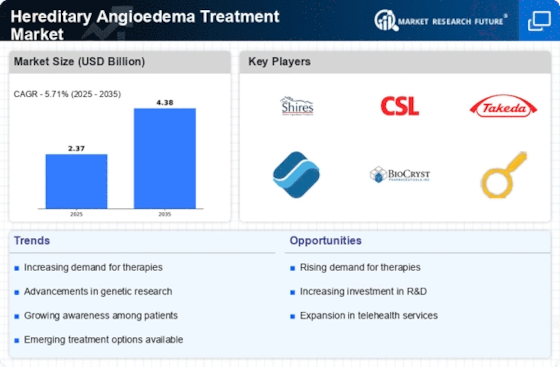
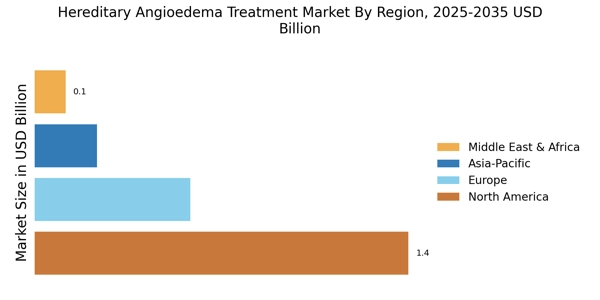
Advancements in Treatment Options
The Hereditary Angioedema Treatment Market is significantly influenced by advancements in treatment options. Recent innovations, including the introduction of targeted therapies and biologics, have transformed the management of HAE. These new treatments not only improve patient outcomes but also reduce the frequency and severity of attacks. For instance, the approval of C1-inhibitor therapies and bradykinin receptor antagonists has provided patients with more effective management strategies. The market is projected to grow as these novel therapies gain traction among healthcare providers and patients alike. Additionally, ongoing clinical trials and research initiatives are likely to yield further advancements, thereby enhancing the therapeutic landscape within the Hereditary Angioedema Treatment Market.
Rising Incidence of Hereditary Angioedema
The Hereditary Angioedema Treatment Market is experiencing growth due to the rising incidence of hereditary angioedema (HAE) cases. Recent estimates suggest that HAE affects approximately 1 in 10,000 to 1 in 50,000 individuals, leading to an increased demand for effective treatment options. As awareness of this condition grows, more patients are being diagnosed, which in turn drives the need for innovative therapies. The increasing prevalence of HAE is prompting healthcare providers to seek advanced treatment modalities, thereby expanding the market. Furthermore, the development of new therapies tailored to specific patient needs is likely to enhance treatment outcomes, contributing to the overall growth of the Hereditary Angioedema Treatment Market.
Growing Patient Advocacy and Support Groups
The presence of patient advocacy and support groups is playing a pivotal role in the Hereditary Angioedema Treatment Market. These organizations are instrumental in raising awareness about HAE, educating patients and healthcare providers, and advocating for better treatment options. Their efforts contribute to increased diagnosis rates and encourage patients to seek appropriate care. Furthermore, these groups often collaborate with pharmaceutical companies to facilitate clinical trials and gather patient feedback, which can influence the development of new therapies. As the influence of these advocacy groups continues to expand, the Hereditary Angioedema Treatment Market is likely to see a corresponding increase in demand for effective treatment solutions.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is a significant driver of the Hereditary Angioedema Treatment Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for novel treatments that address unmet medical needs. This trend is particularly relevant for HAE, where timely access to effective therapies can dramatically improve patient quality of life. The implementation of programs such as orphan drug designations and fast-track approvals is likely to encourage pharmaceutical companies to invest in HAE treatments. As a result, the Hereditary Angioedema Treatment Market is poised for growth, with a steady influx of new therapies that can meet the evolving needs of patients.
Increased Investment in Research and Development
Investment in research and development (R&D) is a crucial driver for the Hereditary Angioedema Treatment Market. Pharmaceutical companies are allocating substantial resources to develop new therapies and improve existing ones. This trend is evident as the market witnesses a surge in clinical trials aimed at exploring innovative treatment modalities. The financial commitment to R&D is expected to yield breakthroughs that could significantly alter the treatment paradigm for HAE. Moreover, collaborations between academic institutions and industry players are fostering an environment conducive to innovation. As a result, the Hereditary Angioedema Treatment Market is likely to benefit from a continuous influx of novel therapies, enhancing patient care and treatment efficacy.