Expansion of Biopharmaceutical Sector
The HCRO Market is experiencing a robust expansion driven by the growth of the biopharmaceutical sector. As biopharmaceutical companies continue to innovate and develop new therapies, the demand for clinical research services is expected to rise correspondingly. Market analysis indicates that the biopharmaceutical industry could reach a valuation of over 500 billion dollars by 2027, creating substantial opportunities for HCROs to support these companies in their research endeavors. This expansion is likely to lead to an increased need for specialized services, including clinical trial management and regulatory support. Furthermore, as biopharmaceutical firms seek to expedite their research processes, they may increasingly rely on HCROs to navigate the complexities of clinical trials. This trend underscores the pivotal role of HCROs in facilitating the growth and success of the biopharmaceutical sector within the HCRO Market.
Regulatory Changes and Compliance Needs
The HCRO Market is significantly influenced by evolving regulatory frameworks and compliance requirements. As governments and regulatory bodies implement stricter guidelines for clinical trials and research practices, the demand for specialized services offered by HCROs is likely to increase. Organizations are compelled to navigate complex regulatory landscapes, which necessitates expertise in compliance and quality assurance. The market for regulatory consulting services within the HCRO sector is projected to grow, as companies seek to mitigate risks associated with non-compliance. Furthermore, the emphasis on patient safety and ethical standards in research is expected to drive the need for HCROs that can ensure adherence to these regulations. This dynamic creates opportunities for HCROs to expand their service offerings and enhance their value proposition in the HCRO Market.
Rising Investment in Healthcare Research
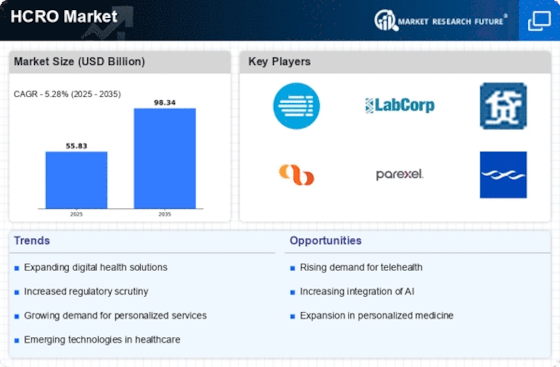
The HCRO Market is experiencing a notable increase in investment directed towards healthcare research. This trend is primarily driven by the growing recognition of the importance of innovative therapies and treatments. In recent years, funding for clinical trials and research initiatives has surged, with estimates indicating that investment in healthcare research could reach upwards of 200 billion dollars by 2026. Such financial backing not only enhances the capabilities of healthcare organizations but also fosters collaboration between various stakeholders, including pharmaceutical companies and research institutions. This collaborative environment is likely to accelerate the development of new drugs and therapies, thereby propelling the HCRO Market forward. As organizations seek to capitalize on these investments, the demand for outsourcing clinical research services is expected to rise, further solidifying the role of HCROs in the healthcare ecosystem.
Growing Focus on Patient-Centric Approaches
The HCRO Market is witnessing a paradigm shift towards patient-centric approaches in clinical research. This trend is characterized by an increasing emphasis on patient engagement and the incorporation of patient feedback into study designs. Organizations are recognizing that understanding patient needs and preferences can lead to more effective treatments and improved outcomes. As a result, HCROs are adapting their methodologies to prioritize patient involvement, which may enhance recruitment and retention rates in clinical trials. Market data suggests that studies incorporating patient perspectives can lead to a 30% increase in participant retention. This focus on patient-centricity not only aligns with the broader healthcare trend of personalized medicine but also positions HCROs as key players in delivering value-driven research solutions within the HCRO Market.
Technological Integration in Clinical Trials
The HCRO Market is increasingly shaped by the integration of advanced technologies in clinical trials. Innovations such as artificial intelligence, big data analytics, and electronic health records are transforming the way research is conducted. These technologies enable more efficient data collection, real-time monitoring, and enhanced patient recruitment strategies. For instance, the use of AI in data analysis can potentially reduce the time required for trial completion by up to 25%. As organizations seek to leverage these technological advancements, the demand for HCROs that can provide expertise in implementing and managing these technologies is likely to grow. This trend not only enhances the efficiency of clinical trials but also improves the overall quality of research outcomes, thereby reinforcing the significance of HCROs in the HCRO Market.