Regulatory Compliance
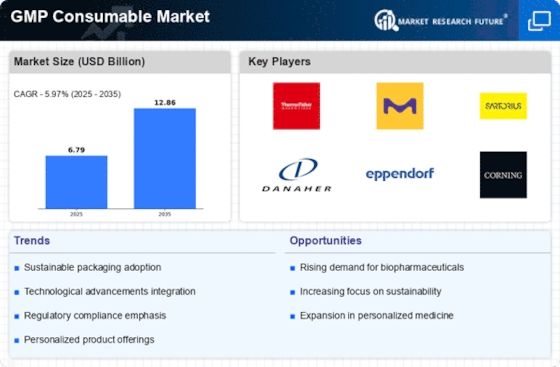
The GMP Consumable Market is heavily influenced by stringent regulatory frameworks that govern the production and distribution of consumables. Compliance with Good Manufacturing Practices is not merely a requirement but a critical driver for market growth. Regulatory bodies, such as the FDA and EMA, enforce guidelines that ensure product safety and efficacy. As a result, companies are compelled to invest in high-quality consumables that meet these standards. The increasing complexity of regulations may lead to a rise in demand for specialized consumables, which could potentially enhance market value. In 2025, the market is projected to reach a valuation of approximately USD 10 billion, driven by the need for compliance and quality assurance in manufacturing processes.
Technological Innovations
Technological innovations play a pivotal role in shaping the GMP Consumable Market. The integration of automation and digital technologies in manufacturing processes enhances efficiency and reduces the risk of contamination. Innovations such as real-time monitoring systems and advanced sterilization techniques are becoming increasingly prevalent. These advancements not only improve product quality but also streamline production timelines, thereby meeting the growing demand for consumables. In 2025, it is anticipated that the market will witness a substantial increase in the adoption of smart technologies, which could potentially lead to a market expansion valued at over USD 12 billion. This trend underscores the importance of technology in driving market growth.
Increased Focus on Quality Assurance
Quality assurance remains a cornerstone of the GMP Consumable Market, as stakeholders prioritize the integrity of their products. The emphasis on quality is driven by consumer expectations and regulatory requirements, compelling manufacturers to adopt rigorous testing and validation processes. This focus on quality assurance not only mitigates risks associated with product recalls but also enhances brand reputation. In 2025, the market is likely to see a shift towards more comprehensive quality management systems, which could result in a market growth rate of approximately 7%. This trend indicates that quality assurance is not merely a regulatory obligation but a strategic advantage in the competitive landscape.
Rising Demand for Biopharmaceuticals
The GMP Consumable Market is experiencing a surge in demand for biopharmaceuticals, which are increasingly recognized for their therapeutic potential. This trend is largely attributed to advancements in biotechnology and a growing focus on personalized medicine. As biopharmaceutical companies expand their product lines, the need for high-quality consumables that adhere to GMP standards becomes paramount. In 2025, the biopharmaceutical sector is expected to account for a significant portion of the consumable market, with estimates suggesting a growth rate of over 8% annually. This rising demand not only propels the market forward but also encourages innovation in consumable products tailored for biopharmaceutical applications.
Sustainability and Environmental Concerns
Sustainability is emerging as a critical driver in the GMP Consumable Market, as companies increasingly recognize the importance of environmentally friendly practices. The demand for sustainable consumables is growing, driven by both regulatory pressures and consumer preferences for eco-friendly products. Manufacturers are exploring biodegradable materials and sustainable sourcing to align with these expectations. In 2025, it is projected that the market for sustainable consumables will expand significantly, potentially reaching USD 5 billion. This shift towards sustainability not only addresses environmental concerns but also positions companies favorably in a market that is progressively valuing corporate responsibility.