Supportive Regulatory Framework
The supportive regulatory environment in Germany plays a crucial role in the growth of the Germany ophthalmic viscoelastic devices market. The European Medicines Agency (EMA) and the Federal Institute for Drugs and Medical Devices (BfArM) have established clear guidelines for the approval and monitoring of ophthalmic products. This regulatory support facilitates the introduction of new viscoelastic devices into the market, ensuring that they meet safety and efficacy standards. As a result, manufacturers are encouraged to invest in research and development, leading to the launch of innovative products. The streamlined approval processes are likely to enhance market accessibility, thereby contributing to the overall growth of the ophthalmic viscoelastic devices sector.
Increasing Healthcare Expenditure
Germany's commitment to healthcare spending is a significant driver for the Germany ophthalmic viscoelastic devices market. The government allocates a substantial portion of its budget to healthcare, which includes funding for advanced medical technologies. In 2025, healthcare expenditure in Germany was projected to reach approximately 12% of the GDP, reflecting a strong emphasis on improving healthcare services. This financial support enables hospitals and clinics to invest in state-of-the-art surgical equipment, including viscoelastic devices, which are essential for modern ophthalmic surgeries. As healthcare budgets continue to grow, the demand for high-quality ophthalmic products is expected to rise, further stimulating market growth.
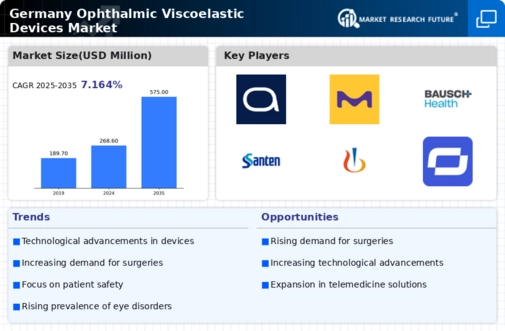
Rising Prevalence of Eye Disorders
The increasing incidence of eye disorders in Germany is a primary driver for the Germany ophthalmic viscoelastic devices market. Conditions such as cataracts and glaucoma are becoming more prevalent, particularly among the aging population. According to recent health statistics, approximately 6 million people in Germany are affected by cataracts, necessitating surgical interventions that utilize viscoelastic devices. This growing patient base is likely to propel demand for advanced surgical tools, including viscoelastic agents, which are essential for maintaining intraocular pressure and protecting ocular tissues during surgery. As the population ages, the need for effective treatment options will continue to rise, thereby fostering growth in the market for ophthalmic viscoelastic devices.
Rising Awareness and Patient Education
The increasing awareness of eye health and the importance of timely surgical interventions are driving the Germany ophthalmic viscoelastic devices market. Public health campaigns and educational initiatives have significantly improved knowledge about eye disorders and available treatment options. As patients become more informed, they are more likely to seek medical advice and undergo necessary surgeries, thereby increasing the demand for viscoelastic devices. Additionally, healthcare providers are actively promoting the benefits of advanced surgical techniques, which further encourages patients to opt for procedures that utilize these devices. This heightened awareness is likely to contribute to the sustained growth of the ophthalmic viscoelastic devices market in Germany.
Technological Innovations in Surgical Procedures
Technological advancements in surgical techniques and equipment are significantly influencing the Germany ophthalmic viscoelastic devices market. Innovations such as minimally invasive surgical methods and the development of new viscoelastic formulations enhance the efficiency and safety of eye surgeries. For instance, the introduction of cohesive and dispersive viscoelastic agents has improved surgical outcomes by providing better control during procedures. The market is expected to witness a compound annual growth rate (CAGR) of around 5% over the next few years, driven by these innovations. Furthermore, the integration of digital technologies in surgical practices is likely to enhance the precision of ophthalmic surgeries, thereby increasing the demand for viscoelastic devices.